Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.18QAP
Related questions
Question
Transcribed Image Text:b Answered: How many atoms of v X
b My Solutions | bartleby
+
Question 2 of 19
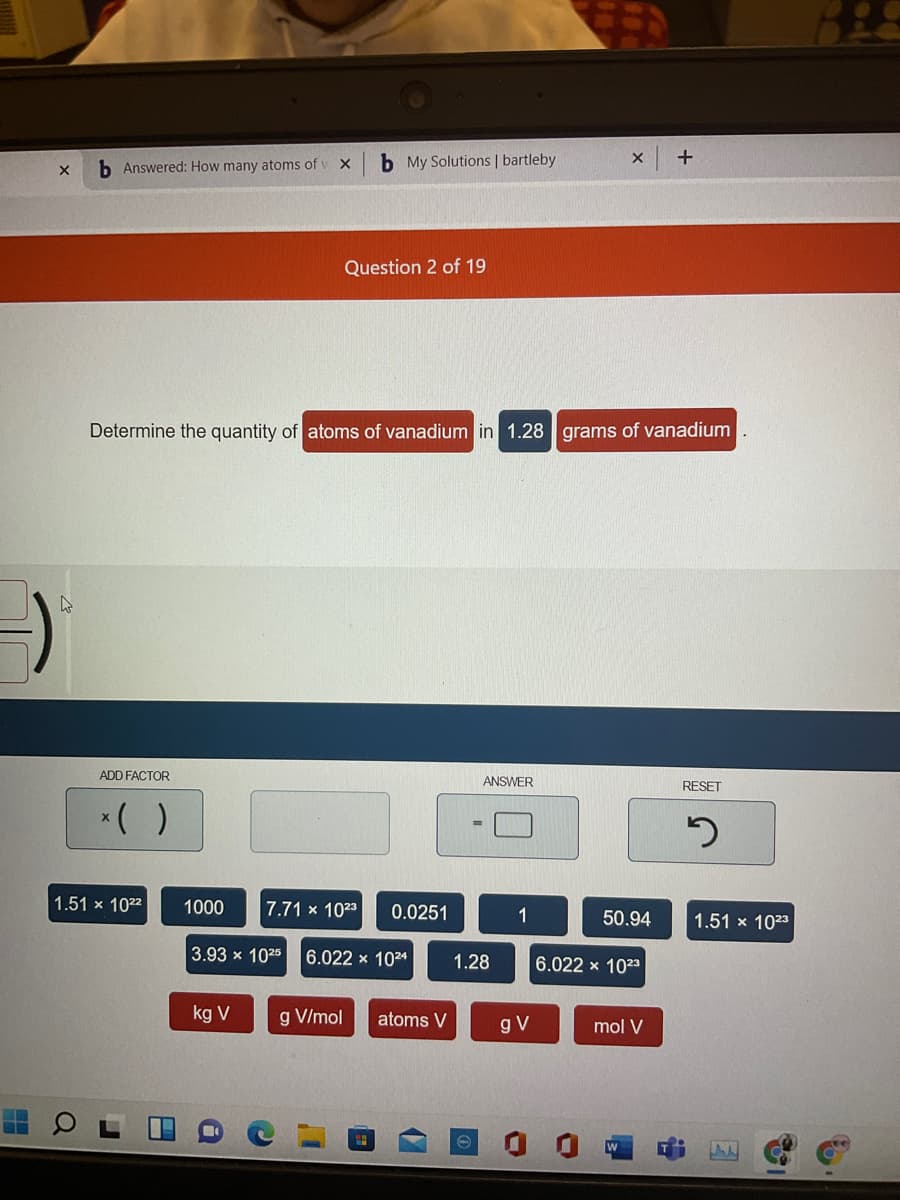
Determine the quantity of atoms of vanadium in 1.28 grams of vanadium
ADD FACTOR
ANSWER
RESET
*( )
1.51 x 1022
1000
7.71 x 1023
0.0251
1
50.94
1.51 x 1023
3.93 x 1025
6.022 x 1024
1.28
6.022 x 1023
kg V
g V/mol
atoms V
gV
mol V
Expert Solution
Step 1
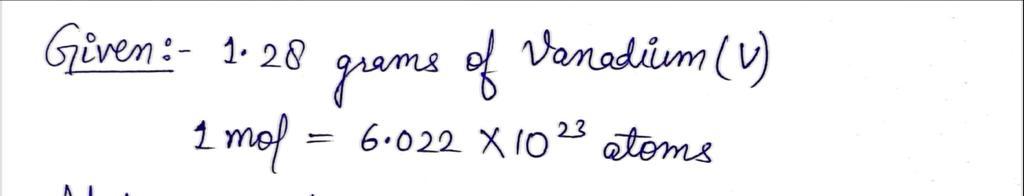
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning