of the balloon is 11.79 L at 40.0 degrees Celsius and a pressure of 153 torr. What volume will the balloon have at standard temperature and pressure (STP)?
of the balloon is 11.79 L at 40.0 degrees Celsius and a pressure of 153 torr. What volume will the balloon have at standard temperature and pressure (STP)?
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter16: Temperature And The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 29P: The mass of a hot-air balloon and its cargo (not including the air inside) is 200 kg. The air...
Related questions
Question
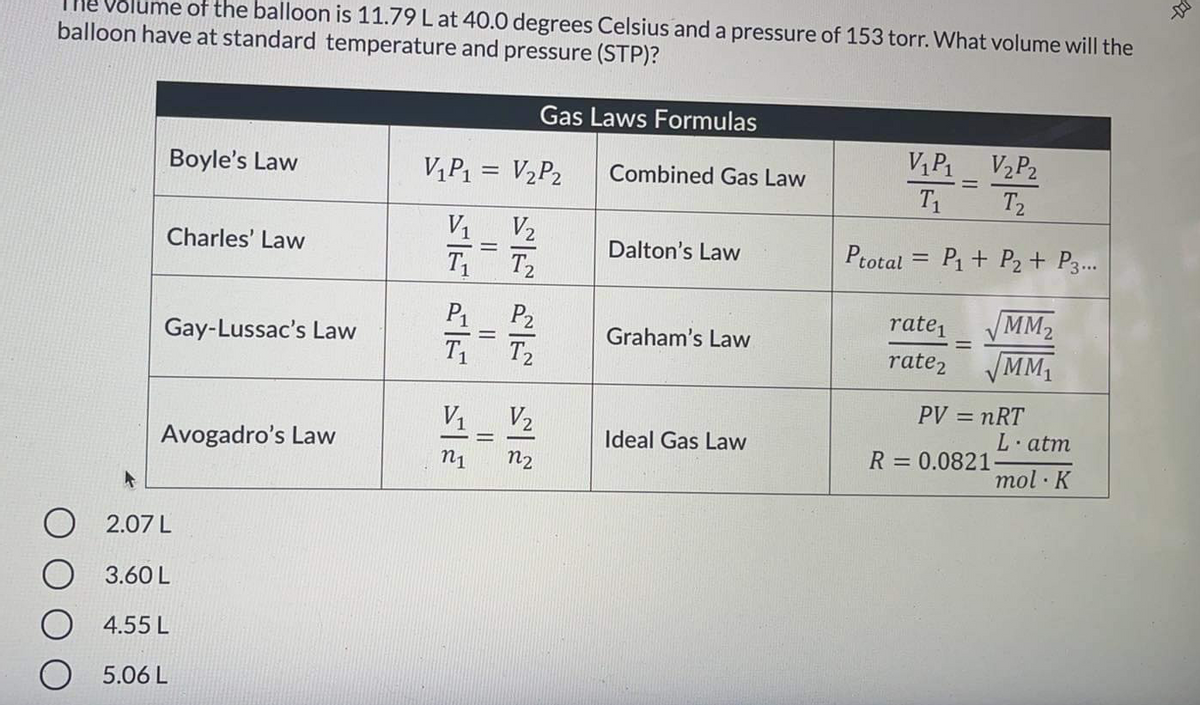
Transcribed Image Text:Volume of the balloon is 11.79 L at 40.0 degrees Celsius and a pressure of 153 torr. What volume will the
balloon have at standard temperature and pressure (STP)?
Gas Laws Formulas
V1P1
V2P2
Boyle's Law
V,P1 = V2P2
Combined Gas Law
%3D
T1
T2
V1 V2
T T
Charles' Law
Dalton's Law
Ptotal = P1 + P2+ P3.
MM2
MM1
P1
P2
rate,
Gay-Lussac's Law
Graham's Law
T T
rate2
V1 V2
PV = nRT
L·atm
%3D
Avogadro's Law
Ideal Gas Law
R = 0.0821-
n1
n2
mol K
O 2.07 L
3.60 L
4.55 L
5.06 L
||
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning