OH TH -H H N H3C- Dess-Martin periodinane provides a means to oxidize a primary alcohol to an aldehyde, while avoiding the use of toxic Cr(VI) compounds. The mechanism involves a reaction similar to the E2 elimination, whereby a C=O double bond is formed with a reduced iodine compound as the leaving group. The reaction occurs in a nonaqueous solvent, CH₂Cl₂, so that the aldehyde product can be recovered. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions HỌ: →XT OAc AcO OAC a d H3C H3C-C-H H ACO OAc -OAC ACO HO CH3 -OACH OAC
OH TH -H H N H3C- Dess-Martin periodinane provides a means to oxidize a primary alcohol to an aldehyde, while avoiding the use of toxic Cr(VI) compounds. The mechanism involves a reaction similar to the E2 elimination, whereby a C=O double bond is formed with a reduced iodine compound as the leaving group. The reaction occurs in a nonaqueous solvent, CH₂Cl₂, so that the aldehyde product can be recovered. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions HỌ: →XT OAc AcO OAC a d H3C H3C-C-H H ACO OAc -OAC ACO HO CH3 -OACH OAC
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 30MP: The carbocation electrophile in a Friede1-Crafts reaction can be generated by an alternate means...
Related questions
Question
#9). Other picture is the instructions on how the arrows must be shown.
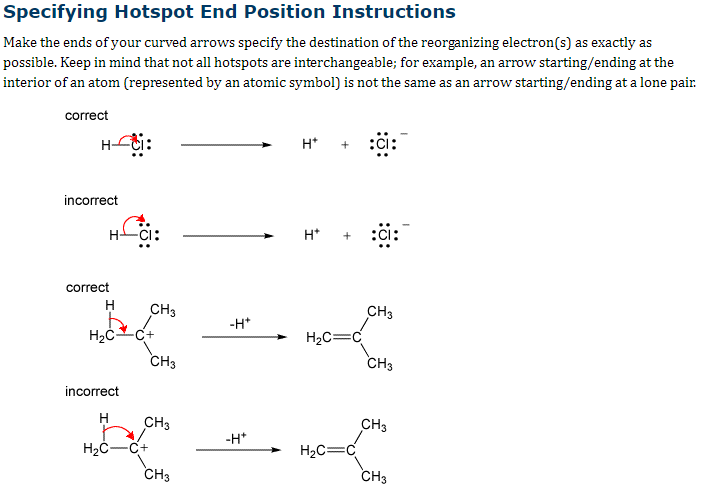
Transcribed Image Text:Specifying Hotspot End Position Instructions
Make the ends of your curved arrows specify the destination of the reorganizing electron(s) as exactly as
possible. Keep in mind that not all hotspots are interchangeable; for example, an arrow starting/ending at the
interior of an atom (represented by an atomic symbol) is not the same as an arrow starting/ending at a lone pair.
correct
H-ti:
incorrect
correct
H
H₂CC+
incorrect
H
CH3
H₂C-C+
CH3
CH3
CH3
-H*
-H*
H*
H*
H₂C=
H₂C=C
CH3
CH3
CH3
CH3
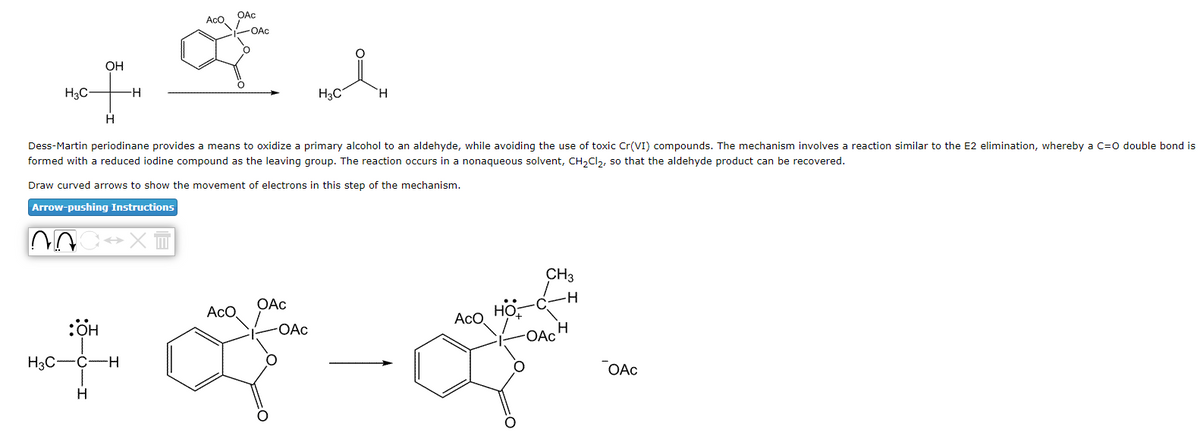
Transcribed Image Text:OH
H3C-
of
H
HO:
H3C-C -H
-H
H
OAc
AcO
-OAC
*
Dess-Martin periodinane provides means to oxidize a primary alcohol to an aldehyde, while avoiding the use of toxic Cr(VI) compounds. The mechanism involves a reaction similar to the E2 elimination, whereby a C=O double bond is
formed with a reduced iodine compound as the leaving group. The reaction occurs in a nonaqueous solvent, CH₂Cl₂, so that the aldehyde product can be recovered.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
AOC XT
ACO
OAc
H3C
-OAc
H
-
Aco
CH3
H
-OAc
H
OAC
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning