OKMNO4 is a secondary standard and it is required to standardized against primary standard. Here, KMNO4 solution is standardized against Sodium Oxalate, Na,CO4. In acidic medium, KMNO, get reduced to Mn2 (aq) while Na,C,O, is being oxidized into CO:(g). The concentration of primary standard which is Na,C,O, has been determined based on the eading of analytical balance, and volume of 250.0 mL KMNO4 . a) Write Oxidation and Reduction half reactions. b) Write the overall balanced Redox reaction. c) What is the Stoichiometric ratio of KMNO4 to Na,CO4.
OKMNO4 is a secondary standard and it is required to standardized against primary standard. Here, KMNO4 solution is standardized against Sodium Oxalate, Na,CO4. In acidic medium, KMNO, get reduced to Mn2 (aq) while Na,C,O, is being oxidized into CO:(g). The concentration of primary standard which is Na,C,O, has been determined based on the eading of analytical balance, and volume of 250.0 mL KMNO4 . a) Write Oxidation and Reduction half reactions. b) Write the overall balanced Redox reaction. c) What is the Stoichiometric ratio of KMNO4 to Na,CO4.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter25: Voltammetry
Section: Chapter Questions
Problem 25.17QAP
Related questions
Question
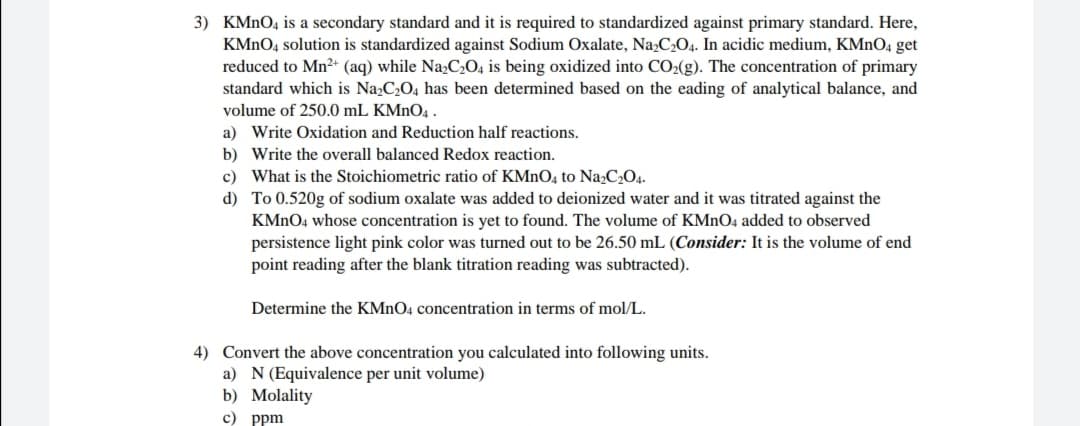
Transcribed Image Text:3) KMNO4 is a secondary standard and it is required to standardized against primary standard. Here,
KMNO4 solution is standardized against Sodium Oxalate, Na,C2O4. In acidic medium, KMNO4 get
reduced to Mn2+ (aq) while Na;C2O, is being oxidized into CO2(g). The concentration of primary
standard which is Na,C2O4 has been determined based on the eading of analytical balance, and
volume of 250.0 mL KMNO4 .
a) Write Oxidation and Reduction half reactions.
b) Write the overall balanced Redox reaction.
c) What is the Stoichiometric ratio of KMNO4 to Na2C2O4.
d) To 0.520g of sodium oxalate was added to deionized water and it was titrated against the
KMNO4 whose concentration is yet to found. The volume of KMNO4 added to observed
persistence light pink color was turned out to be 26.50 mL (Consider: It is the volume of end
point reading after the blank titration reading was subtracted).
Determine the KMNO4 concentration in terms of mol/L.
4) Convert the above concentration you calculated into following units.
a) N(Equivalence per unit volume)
b) Molality
с) ppm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
