On a spacecraft, the COp the crew exhales is removed from the cabin by a reaction within canisters of lithium hydroxide (LIOH). COp + 2 LIOH - LiCO + H;O Determine the amount of LiOHa required for a seven-day mission in space with three astronauts. Assume each astronaut expels 880 grams of CO per day.
On a spacecraft, the COp the crew exhales is removed from the cabin by a reaction within canisters of lithium hydroxide (LIOH). COp + 2 LIOH - LiCO + H;O Determine the amount of LiOHa required for a seven-day mission in space with three astronauts. Assume each astronaut expels 880 grams of CO per day.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 120E: The space shuttle environmental control system handles excess CO2 (which the astronauts breathe out;...
Related questions
Question
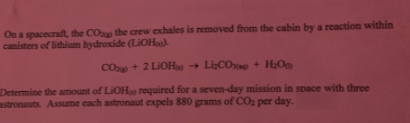
Transcribed Image Text:On a spacecraft, the COp the crew exhales is removed from the cabin by a reaction within
canisters of lithium hydroxide (LIOH).
COp + 2 LIOH
- LiCO + H;O
Determine the amount of LiOHa required for a seven-day mission in space with three
astronauts. Assume each astronaut expels 880 grams of CO per day.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning