On another planet, the isotopes of titanium have the given natural abundances. Abundance Mass (u) 70.200% 45.95263 10.600% 47.94795 19.200% 49.94479 Isotope 46 Ti 48 Ti 50 Ti What is the average atomic mass of titanium on that planet?
On another planet, the isotopes of titanium have the given natural abundances. Abundance Mass (u) 70.200% 45.95263 10.600% 47.94795 19.200% 49.94479 Isotope 46 Ti 48 Ti 50 Ti What is the average atomic mass of titanium on that planet?
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 22E: An element has the following natural abundances and isotopic masses: 90.92% abundance with 19.99...
Related questions
Question
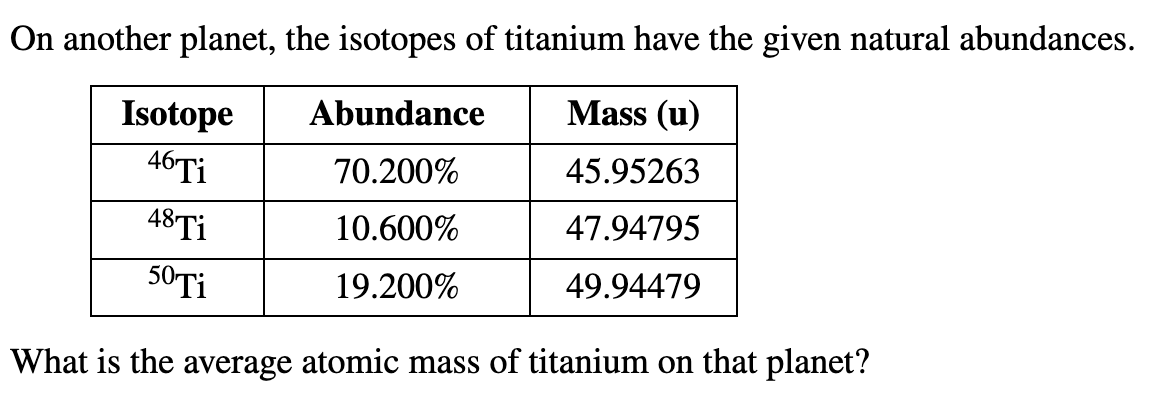
Transcribed Image Text:On another planet, the isotopes of titanium have the given natural abundances.
Abundance
Mass (u)
70.200%
45.95263
10.600%
47.94795
19.200%
49.94479
Isotope
46 Ti
48 Ti
50 Ti
What is the average atomic mass of titanium on that planet?
Expert Solution
Step 1
1.Average atomic mass is the weighted average mass of the sample that occure in nature.
2. Here the element is Ti
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
Expert Answers to Latest Homework Questions
Q: QUESTION 6
Based on the following processes shown below, which of the following statements is true?…
Q: Help please
Q: The following endothermic reaction is at equilibrium in a closed vessel.…
Q: QUESTION 5
What two particles reside in the nucleus?
protons, electrons
futons, protons
protons,…
Q: Question Completion Status:
QUESTION 11
Take Test: Exam #4
happens in the mitochondria, whereas…
Q: Problem 4 (30 points)
Amy is interested in moving to California but isn't certain
which city she'd…
Q: Can someone help me with this question from my homework assp?
A pharmacist wants to mix a 45%…
Q: 1 What are the main powers and responsibilities of the U.S. Supreme Court within the American…
Q: a) Calcuated the peak-to-peak values of the voltage V1, V2, V3
I got:
V1 = 3.81V∠29.61°
V2 =…
Q: Take Test: Exam #4
Question Completion Status:
QUESTION 10
Which of the following choices requires…
Q: Can someone help me solve this system by using the substitution method
y=-(x-2)^2 +3
y=7x+1
Also…
Q: A credit score is a numerical value between 300 and 850 that measures a person's creditworthiness.…
Q: QUESTION 1
How many molecules of C2H5OH (density = 0.789 g/mL) are there in 50.0 mL?
O 1.29 x 1023…
Q: Old Faithful, the most reliable geyser in Yellowstone National Park can shoot up to 8,400
gallons of…
Q: If the equilibrium constant for the reaction below is equal to 1x10^-14. 2H2O(l) <-->H3O+(aq)…
Q: Can someone please help? Thanks
Q: Income statement and balance sheet data for The Athletic Attic are provided below.
THE ATHLETIC…
Q: Question Completion Status:
QUESTION 8
Take Test: Exam #4
Which is not a function of your skeleton…
Q: Income statement and balance sheet data for Great Adventures, Incorporated, are provided below.…
Q: Income statement and balance sheet data for Great Adventures, Incorporated, are provided below.…
Q: Question Completion Status:
QUESTION 22
Take Test: Exam #4
According to this figure, how do hydrogen…