One car is traveling at 60.0 miles per hour, another car is traveling at 100.0 kilometers per hour. Determine which car is traveling faster by converting both speeds to feet per second. (There are 5280 feet in a mile. Round your answers to one decimal place after the decimal).
One car is traveling at 60.0 miles per hour, another car is traveling at 100.0 kilometers per hour. Determine which car is traveling faster by converting both speeds to feet per second. (There are 5280 feet in a mile. Round your answers to one decimal place after the decimal).
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1ALQ: a. There are 365 days per year, 24 hours per day, 12 months per year, and 60 minutes per hour. Use...
Related questions
Question
One car is traveling at 60.0 miles per hour, another car is traveling at 100.0 kilometers per hour. Determine which car is traveling faster by converting both speeds to feet per second. (There are 5280 feet in a mile. Round your answers to one decimal place after the decimal).
Expert Solution
Step 1
Converting the speed of first car from miles per hour to feet per second:
1 mile = 5280 feet
1 hour = 3600 seconds
Thus, 60 miles per hour can be converted to feet per second as: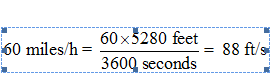
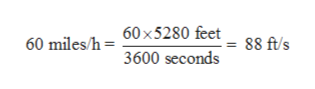
Step 2
Converting the speed of second car from km//h to ft/s:
1 km = 3280.84 ft
1 h = 3600 s
Thus, 100 km/h can be converted to m/s as:

Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning