One day in lab, while adding a gnarled root to a dark liquid bubbling in an iron cauldron, your friend Carmen (an expert chemist) says this: "Metal sulfides roasted with oxygen produce the corresponding oxide and sulfur dioxide gas." Using Carmen's statement, and what you already know about chemistry, predict the products of the following reaction. Be sure your chemical equation is balanced! Ag,8 (s) + O,(2) → I NO
One day in lab, while adding a gnarled root to a dark liquid bubbling in an iron cauldron, your friend Carmen (an expert chemist) says this: "Metal sulfides roasted with oxygen produce the corresponding oxide and sulfur dioxide gas." Using Carmen's statement, and what you already know about chemistry, predict the products of the following reaction. Be sure your chemical equation is balanced! Ag,8 (s) + O,(2) → I NO
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section: Chapter Questions
Problem 58QRT: Hydrazine, N2H4, has been proposed as the fuel in a fuel cell in which oxygen is the oxidizing...
Related questions
Question
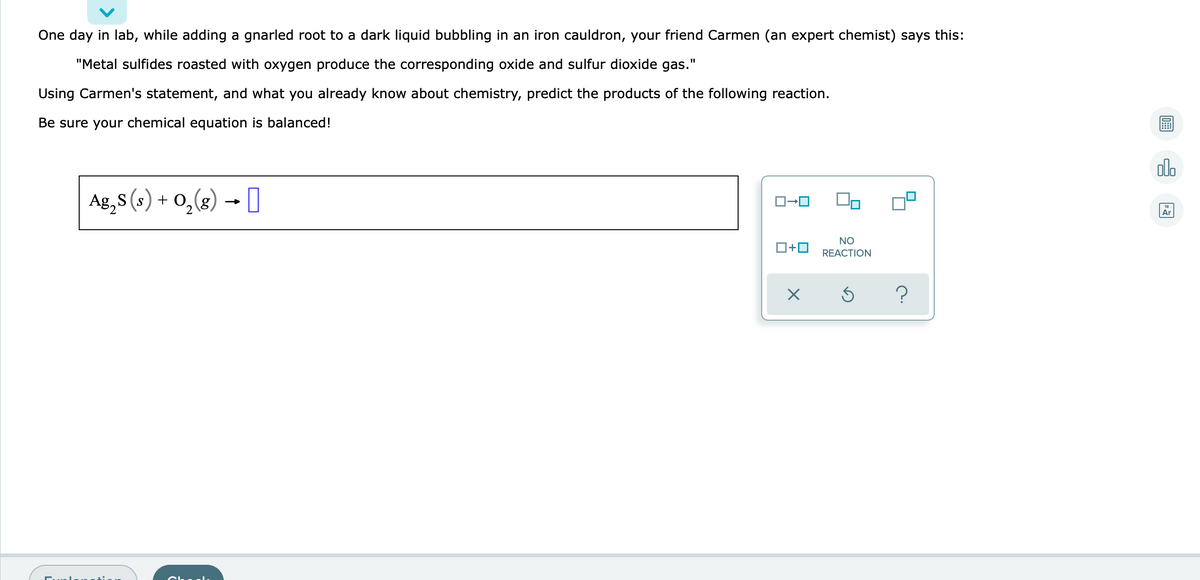
Transcribed Image Text:One day in lab, while adding a gnarled root to a dark liquid bubbling in an iron cauldron, your friend Carmen (an expert chemist) says this:
"Metal sulfides roasted with oxygen produce the corresponding oxide and sulfur dioxide gas."
Using Carmen's statement, and what you already know about chemistry, predict the products of the following reaction.
Be sure your chemical equation is balanced!
olo
Ag,s (s) → [
+ 0,(8)
2
Ar
NO
O+0
REACTION
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning