One molecule of water (H2O) contains two atoms of hydrogen and one atom of oxygen. A hydrogen atom has a mass of 1.0 u and an atom of oxygen has a mass of 16 u, approximately. (a) What is the mass of one molecule of water? (b) How many molecules of water are in the world's oceans, which have an estimated total mass of 1.4 x 1021 kg? (a) Number i Units (b) Number i Units
One molecule of water (H2O) contains two atoms of hydrogen and one atom of oxygen. A hydrogen atom has a mass of 1.0 u and an atom of oxygen has a mass of 16 u, approximately. (a) What is the mass of one molecule of water? (b) How many molecules of water are in the world's oceans, which have an estimated total mass of 1.4 x 1021 kg? (a) Number i Units (b) Number i Units
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter1: Getting Started
Section: Chapter Questions
Problem 48PQ: In 2011, artist Hans-Peter Feldmann covered the walls of a gallery at the New York Guggenheim Museum...
Related questions
Question
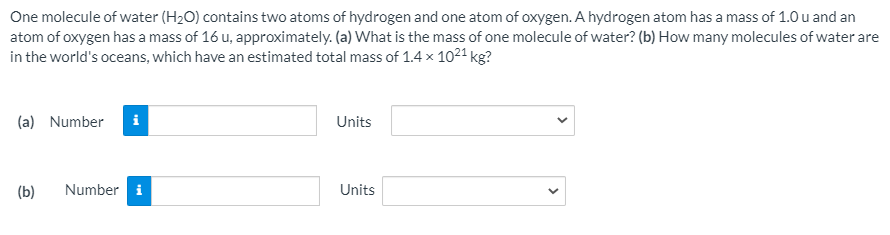
Transcribed Image Text:One molecule of water (H2O) contains two atoms of hydrogen and one atom of oxygen. A hydrogen atom has a mass of 1.0 u and an
atom of oxygen has a mass of 16 u, approximately. (a) What is the mass of one molecule of water? (b) How many molecules of water are
in the world's oceans, which have an estimated total mass of 1.4 x 1021 kg?
(a) Number
i
Units
(b)
Number i
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College