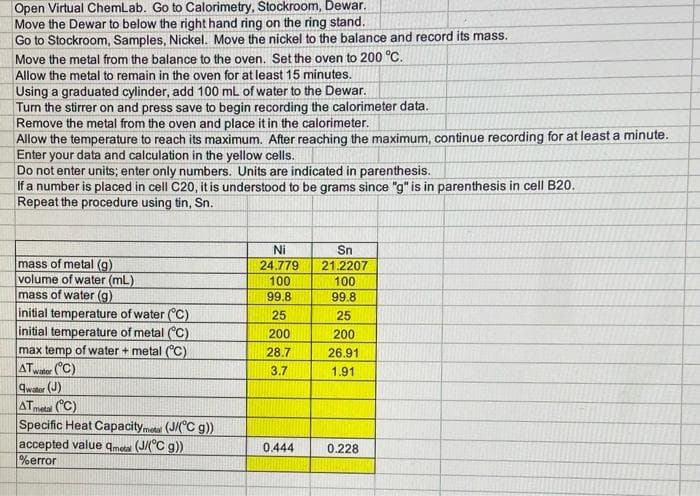
Open Virtual ChemLab. Go to Calorimetry, Stockroom, Dewar. Move the Dewar to below the right hand ring on the ring stand. Go to Stockroom, Samples, Nickel. Move the nickel to the balance and record its mass. Move the metal from the balance to the oven. Set the oven to 200 °C. Allow the metal to remain in the oven for at least 15 minutes. Using a graduated cylinder, add 100 mL of water to the Dewar. Turn the stirrer on and press save to begin recording the calorimeter data. Remove the metal from the oven and place it in the calorimeter. Allow the temperature to reach its maximum. After reaching the maximum, continue recording for at least a minute. Enter your data and calculation in the yellow cells. Do not enter units; enter only numbers. Units are indicated in parenthesis. If a number is placed in cell C20, it is understood to be grams since "g" is in parenthesis in cell B20. Repeat the procedure using tin, Sn. mass of metal (g) volume of water (mL) mass of water (g) initial temperature of water (C) initial temperature of metal (C) max temp of water + metal (C) ATwater (C) Quater (J) ATmeta (C) Specific Heat Capacityme (J/C g)) accepted value qmeta (J/(°C g)) %error Ni 24.779 100 99.8 25 200 28.7 3.7 0.444 Sn 21.2207 100 99.8 25 200 26.91 1.91 0.228
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
please help on following blanks. Also I dont know what the mass of water is but assumed maybe 99.8
Step by step
Solved in 6 steps with 7 images


