orksheet 113 - Specific Heat - 3400646 - Saved 11 ont References Review x Aav v JS.) View AAAEEEEE A = Help in EET Paragraph Text Predictions: On Editor Suggestions: Showing Fy P Search (Alt+Q) AaBbCc Normal AaBbCc No Spacing I Styles AaBbCc Heading 1 Comments 4. The heat capacity of aluminum is 0.900 J/gÇ. a. How much energy is needed to raise the temperature of an.8.50 x 10² g block of aluminum from 22.8°C to 94.6°C? Find Replace Editing Reus Files Reuse Fi L
orksheet 113 - Specific Heat - 3400646 - Saved 11 ont References Review x Aav v JS.) View AAAEEEEE A = Help in EET Paragraph Text Predictions: On Editor Suggestions: Showing Fy P Search (Alt+Q) AaBbCc Normal AaBbCc No Spacing I Styles AaBbCc Heading 1 Comments 4. The heat capacity of aluminum is 0.900 J/gÇ. a. How much energy is needed to raise the temperature of an.8.50 x 10² g block of aluminum from 22.8°C to 94.6°C? Find Replace Editing Reus Files Reuse Fi L
College Physics
10th Edition
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter11: Energy In Thermal Processes
Section: Chapter Questions
Problem 15P: What mass of water at 25.0C must be allowed to conic to thermal equilibrium with a 1.85-kg cube of...
Related questions
Question
Transcribed Image Text:orksheet 113 - Specific Heat - 3400646 - Saved
ont
References Review
11
X² Aav - A
@
F2
A A A EEEEE¶<
三三三
J.S.) Text Predictions: On Editor Suggestions: Showing
J
F3
#
3
View
DII
Help
$
Paragraph
2.54
4
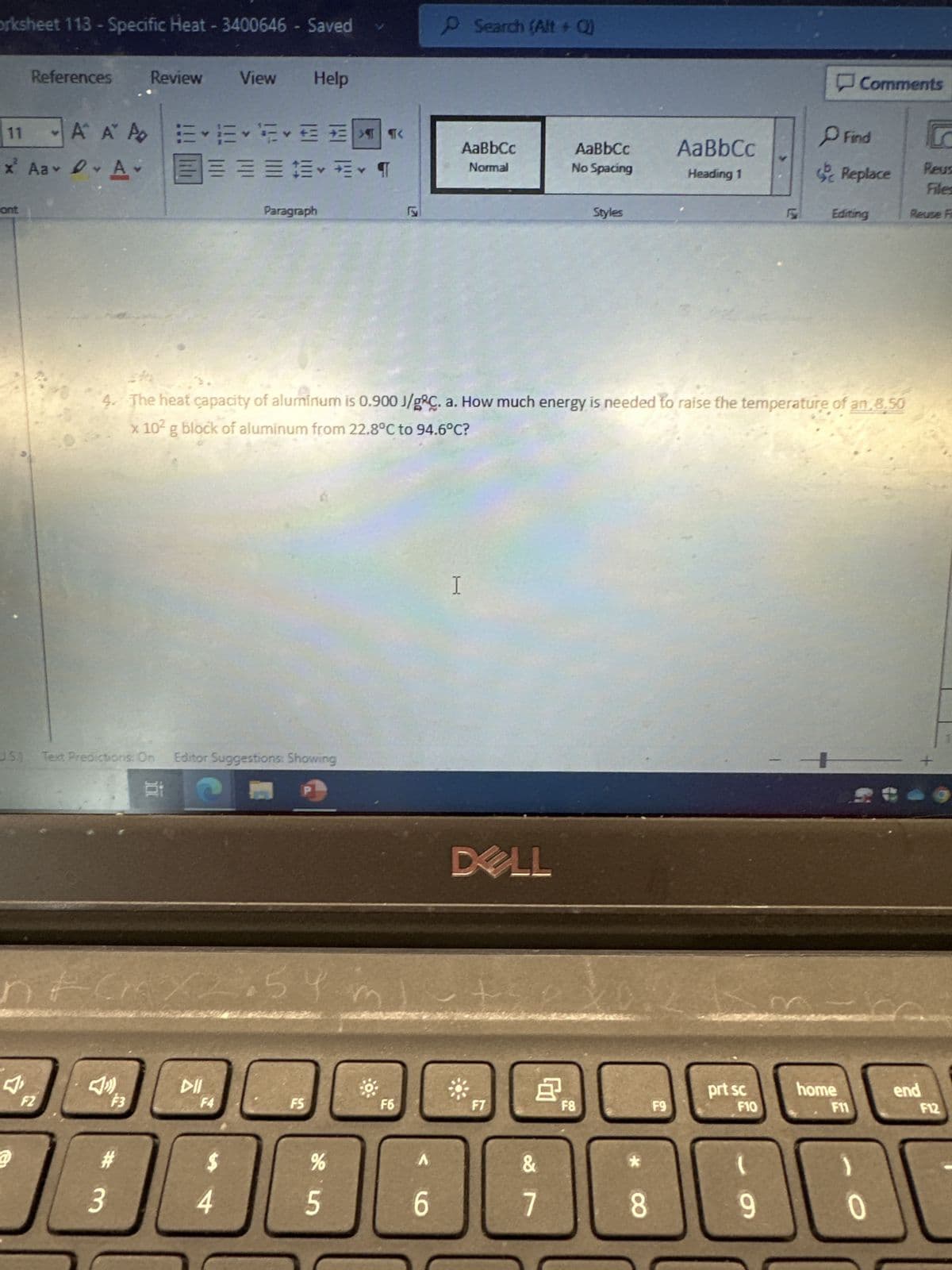
4. The heat capacity of aluminum is 0.900 J/gÇ. a. How much energy is needed to raise the temperature of an 8.50
x 10² g block of aluminum from 22.8°C to 94.6°C?
F5
%
5
7
F6
P Search (Alt + Q)
A
J-
6
AaBbCc
Normal
I
DALL
*
+
F7
8
AaBbC
No Spacing
&
7
Styles
F8
*
8
AaBbCc
Heading 1
F9
prt sc
F10
(
9
Comments
Find
Replace
Editing
home
F11
0
Reus
Files
Reuse Fa
end
F12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning