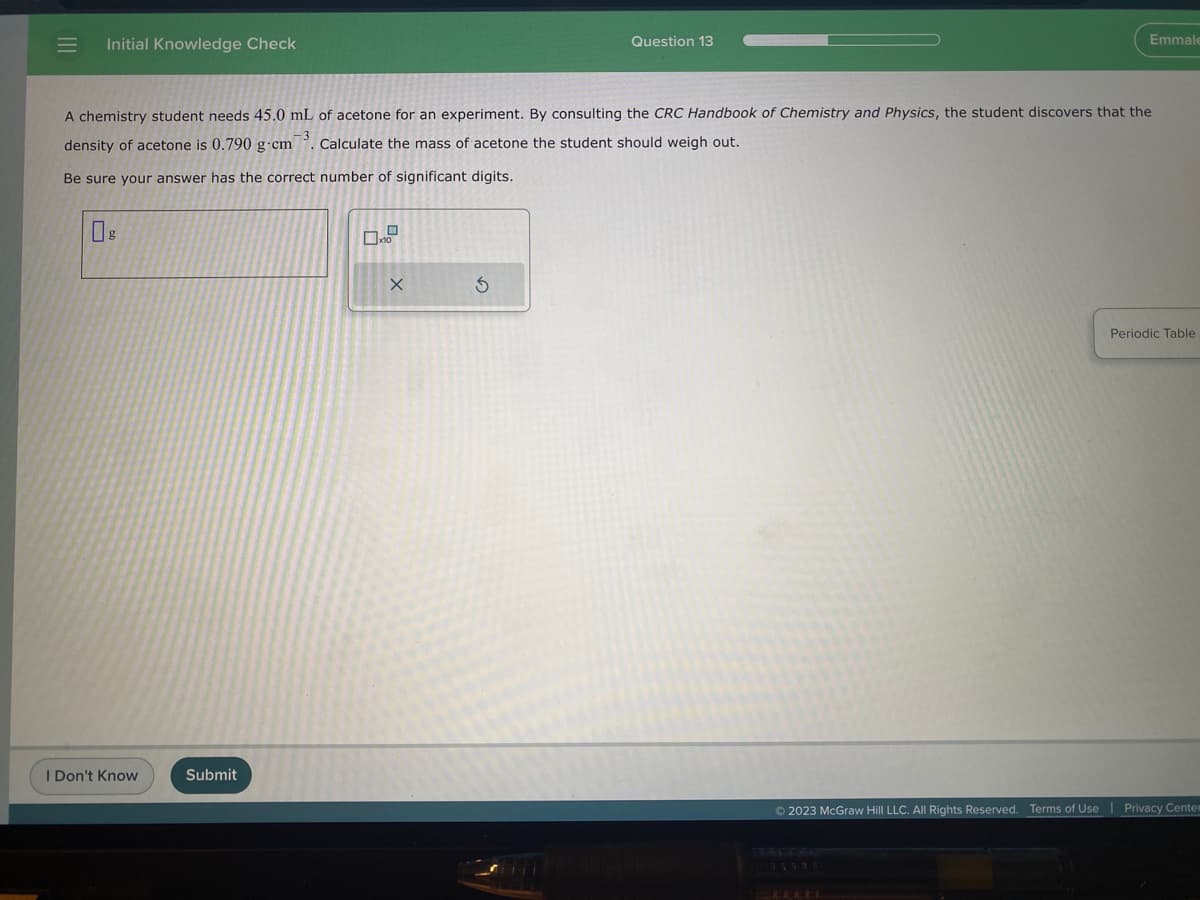
||| = Initial Knowledge Check g A chemistry student needs 45.0 mL of acetone for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers that the -3 density of acetone is 0.790 g-cm Calculate the mass acetone the student should weigh out. Be sure your answer has the correct number of significant digits. X Question 13 S Emmale
||| = Initial Knowledge Check g A chemistry student needs 45.0 mL of acetone for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers that the -3 density of acetone is 0.790 g-cm Calculate the mass acetone the student should weigh out. Be sure your answer has the correct number of significant digits. X Question 13 S Emmale
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 21RPS: You and your lab partner are asked to determine the density of an aluminum bar. The mass is known...
Related questions
Question
Transcribed Image Text:|||
=
Initial Knowledge Check
I Don't Know
A chemistry student needs 45.0 mL of acetone for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers that the
density of acetone is 0.790 g-cm. Calculate the mass of acetone the student should weigh out.
Be sure your answer has the correct number of significant digits.
Submit
x
O
X
Question 13
S
Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use
Emmale
ELLEL
Periodic Table
Privacy Centem
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning