Part A a solution that is 0.19 M in HCHO2 and 0.13 M in NaCHO2 (Ka(HCHO2) = 1.8 × 10-4) Express your answer to two decimal places. ΑΣφ ? pH Submit Request Answer Part B a solution that is 0.16 M in NH3 and 0.17 M in NH, Cl (Kp (NH3) = 1.76 × 10–5) Express your answer to two decimal places. | ν ΑΣφ ? pH = Notes
Part A a solution that is 0.19 M in HCHO2 and 0.13 M in NaCHO2 (Ka(HCHO2) = 1.8 × 10-4) Express your answer to two decimal places. ΑΣφ ? pH Submit Request Answer Part B a solution that is 0.16 M in NH3 and 0.17 M in NH, Cl (Kp (NH3) = 1.76 × 10–5) Express your answer to two decimal places. | ν ΑΣφ ? pH = Notes
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 6P
Related questions
Question
5. please answer a and b
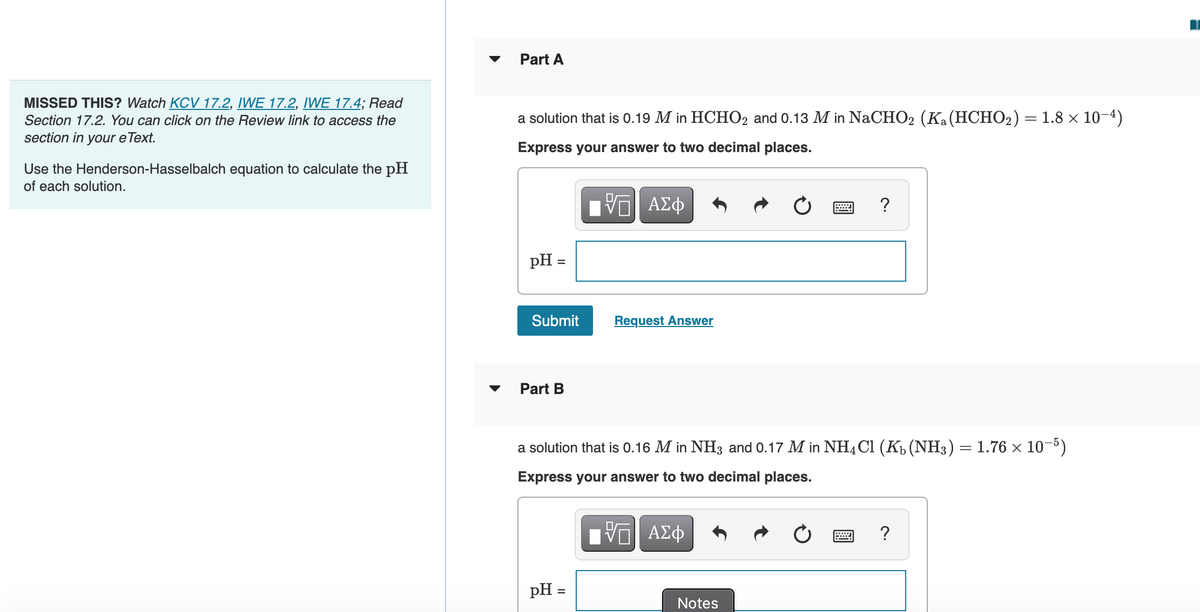
Transcribed Image Text:Part A
MISSED THIS? Watch KCV 17.2, IWE 17.2, IWE 17.4; Read
Section 17.2. You can click on the Review link to access the
a solution that is 0.19 M in HCHO2 and 0.13 M in NaCHO2 (Ka(HCHO2) = 1.8 × 10-4)
section in your eText.
Express your answer to two decimal places.
Use the Henderson-Hasselbalch equation to calculate the pH
of each solution.
Hν ΑΣφ
pH =
Submit
Request Answer
Part B
a solution that is 0.16 M in NH3 and 0.17 M in NHạ Cl (Kp (NH3) = 1.76 x 10-5)
Express your answer to two decimal places.
Hν ΑΣφ
?
pH =
Notes
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

