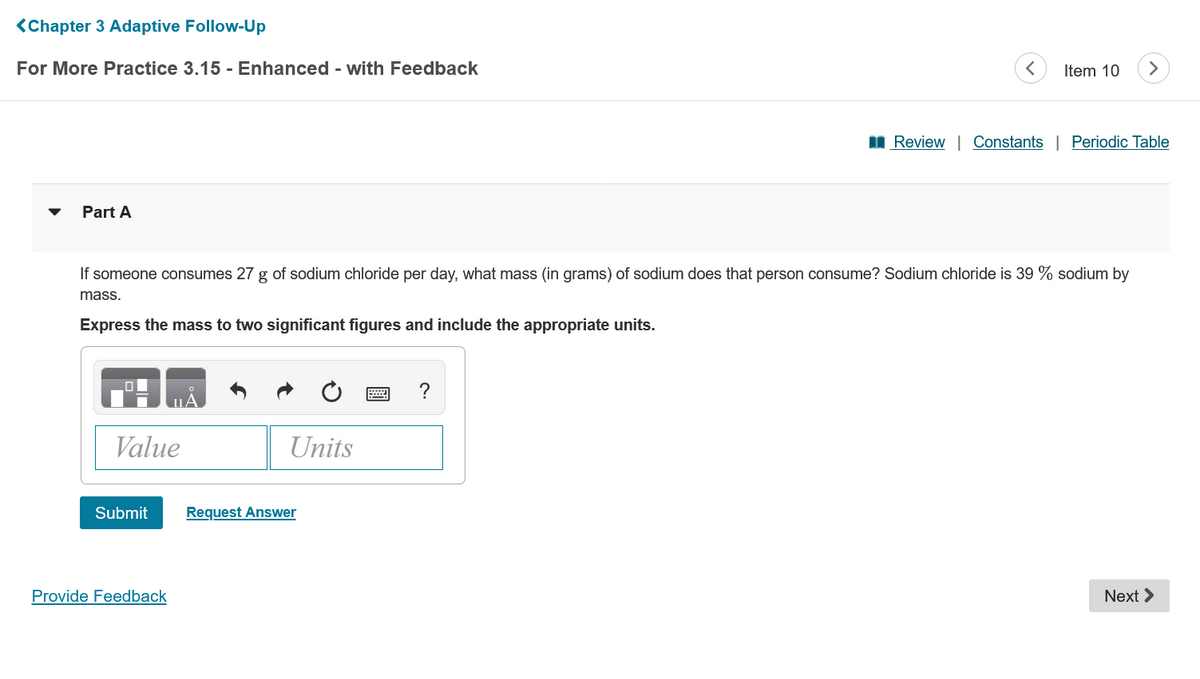
Part A If someone consumes 27 g of sodium chloride per day, what mass (in grams) of sodium does that person consume? Sodium chloride is 39 % sodium b mass. Express the mass to two significant figures and include the appropriate units. ? Value Units Submit Request Answer
Part A If someone consumes 27 g of sodium chloride per day, what mass (in grams) of sodium does that person consume? Sodium chloride is 39 % sodium b mass. Express the mass to two significant figures and include the appropriate units. ? Value Units Submit Request Answer
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 36QAP: At sea, distances are measured in nautical miles and speeds are expressed in knots. 1 nautical mile...
Related questions
Question
5
Transcribed Image Text:<Chapter 3 Adaptive Follow-Up
For More Practice 3.15 - Enhanced - with Feedback
Item 10
>
I Review | Constants | Periodic Table
Part A
If someone consumes 27 g of sodium chloride per day, what mass (in grams) of sodium does that person consume? Sodium chloride is 39 % sodium by
mass.
Express the mass to two significant figures and include the appropriate units.
?
Value
Units
Submit
Request Answer
Provide Feedback
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning