Part A Rank the following five elements by ionization energy. Rank from highest to lowest ionization energy. To rank items as equivalent, overlap them. Part B Rank the following elements by ionization energy. Rank from highest to lowest ionization energy. To rank items as equivalent, overlap them.
Part A Rank the following five elements by ionization energy. Rank from highest to lowest ionization energy. To rank items as equivalent, overlap them. Part B Rank the following elements by ionization energy. Rank from highest to lowest ionization energy. To rank items as equivalent, overlap them.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.68QE
Related questions
Question
Part A
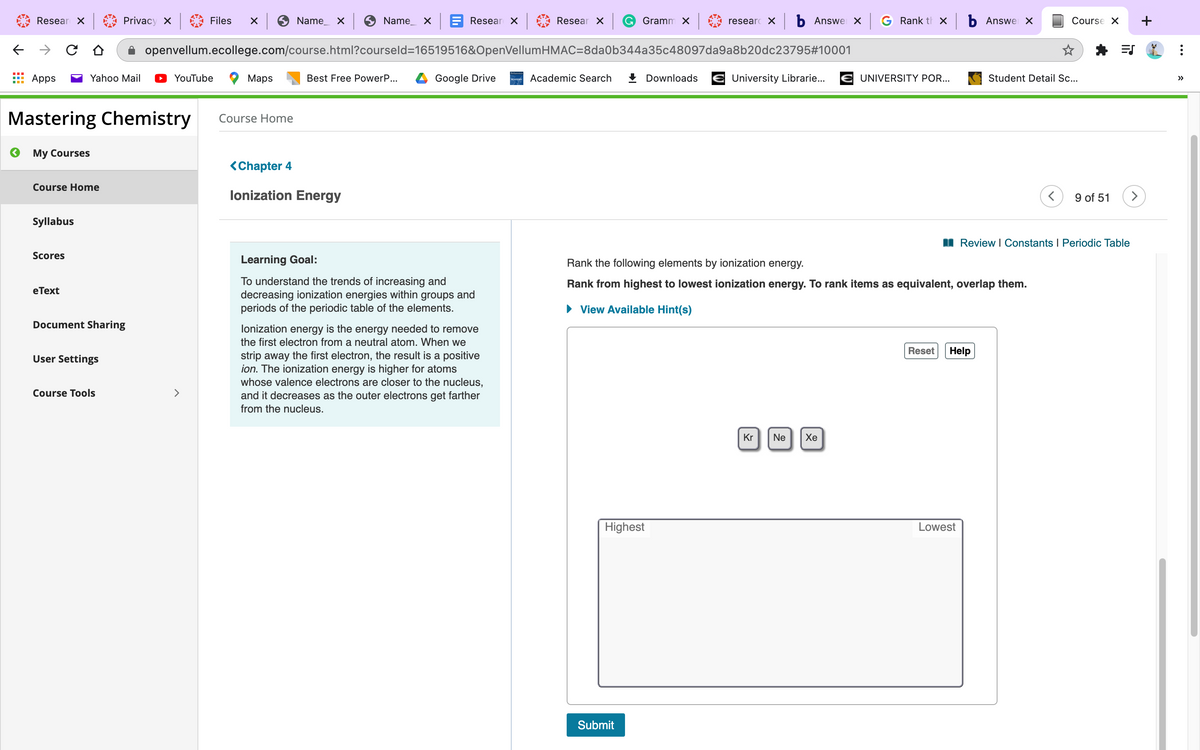
Rank the following five elements by ionization energy.
Rank from highest to lowest ionization energy. To rank items as equivalent, overlap them.
Part B
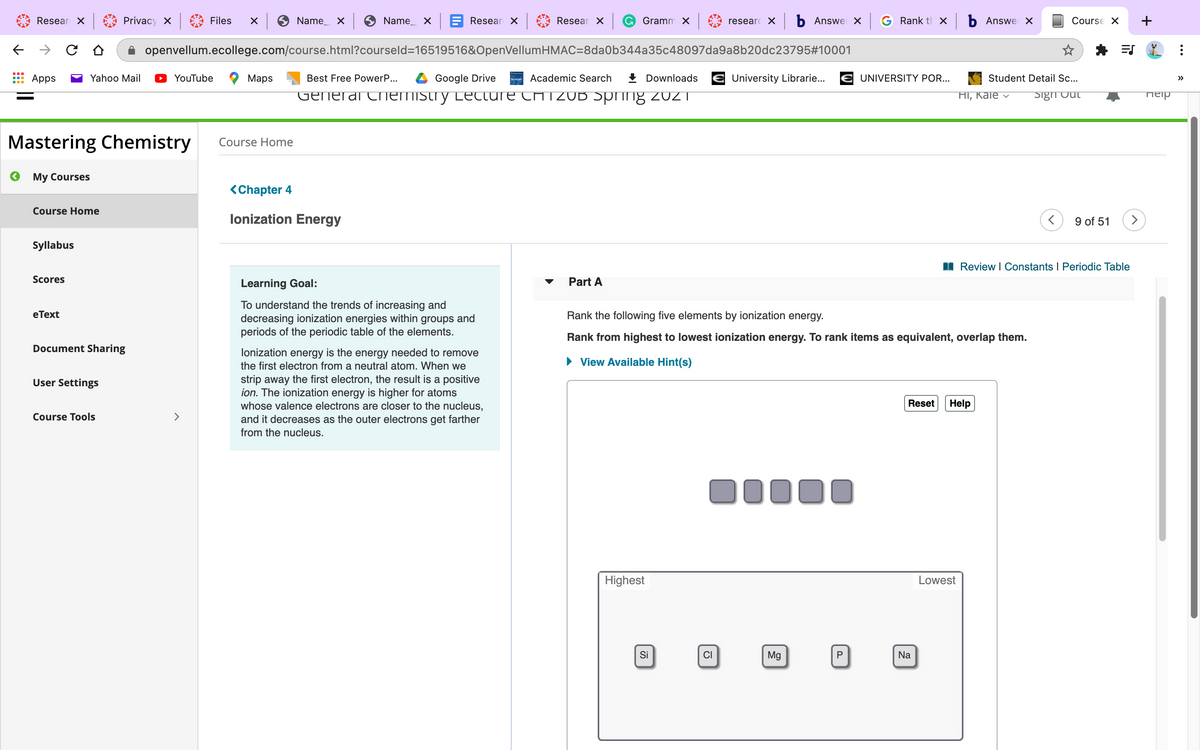
Rank the following elements by ionization energy.
Rank from highest to lowest ionization energy. To rank items as equivalent, overlap them.
Transcribed Image Text:Resear X
Privacy x
Files
Name_ X
Name_ X
Resear X
Resear X
Gramm X
researc X b Answe X
G Rank t X
b Answe X
Course X
+
openvellum.ecollege.com/course.html?courseld=16519516&OpenVellumHMAC=8da0b344a35c48097da9a8b20dc23795#10001
Apps
Yahoo Mail
YouTube
Мaps
Best Free PowerP...
Google Drive
Academic Search
+ Downloads
University Librarie...
E UNIVERSITY POR...
Student Detail Sc..
>>
Mastering Chemistry
Course Home
My Courses
<Chapter 4
Course Home
lonization Energy
9 of 51
Syllabus
I Review I Constants I Periodic Table
Scores
Learning Goal:
Rank the following elements by ionization energy.
To understand the trends of increasing and
decreasing ionization energies within groups and
periods of the periodic table of the elements.
Rank from highest to lowest ionization energy. To rank items as equivalent, overlap them.
eТext
• View Available Hint(s)
Document Sharing
lonization energy is the energy needed to remove
the first electron from a neutral atom. When we
Reset
Help
strip away the first electron, the result is a positive
ion. The ionization energy is higher for atoms
whose valence electrons are closer to the nucleus,
and it decreases as the outer electrons get farther
from the nucleus.
User Settings
Course Tools
>
Kr
Ne
Хе
Highest
Lowest
Submit
Transcribed Image Text:Resear X
Privacy X
Files
Name_ X
Name_ X
Resear X
Resear X
Gramm X
researc X b Answe X
G Rank t X
b Answe X
Course X
+
openvellum.ecollege.com/course.html?courseld=16519516&OpenVellumHMAC=8da0b344a35c48097da9a8b20dc23795#10001
Apps
Yahoo Mail
YouTube
Мaps
Best Free PowerP...
Google Drive
Academic Search
! Downloads
University Librarie...
E UNIVERSITY POR...
Student Detail .c..
>>
Generai CNemistry LEcture CHTZUB SPing 2021
HI, Kale ♥
Sign Out
петр
Mastering Chemistry
Course Home
My Courses
<Chapter 4
Course Home
lonization Energy
9 of 51
Syllabus
I Review I Constants I Periodic Table
Scores
Learning Goal:
Part A
To understand the trends of increasing and
decreasing ionization energies within groups and
periods of the periodic table of the elements.
eТext
Rank the following five elements by ionization energy.
Rank from highest to lowest ionization energy. To rank items as equivalent, overlap them.
Document Sharing
lonization energy is the energy needed to remove
the first electron from a neutral atom. When we
• View Available Hint(s)
strip away the first electron, the result is a positive
ion. The ionization energy is higher for atoms
whose valence electrons are closer to the nucleus,
and it decreases as the outer electrons get farther
User Settings
Reset
Help
Course Tools
>
from the nucleus.
D0000
Highest
Lowest
Si
Mg
CI
P
Na
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning