Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 76E: Water gas, a mixture of H2 and CO, is an important industrial fuel produced by the reaction of steam...
Related questions
Concept explainers
Question
100%
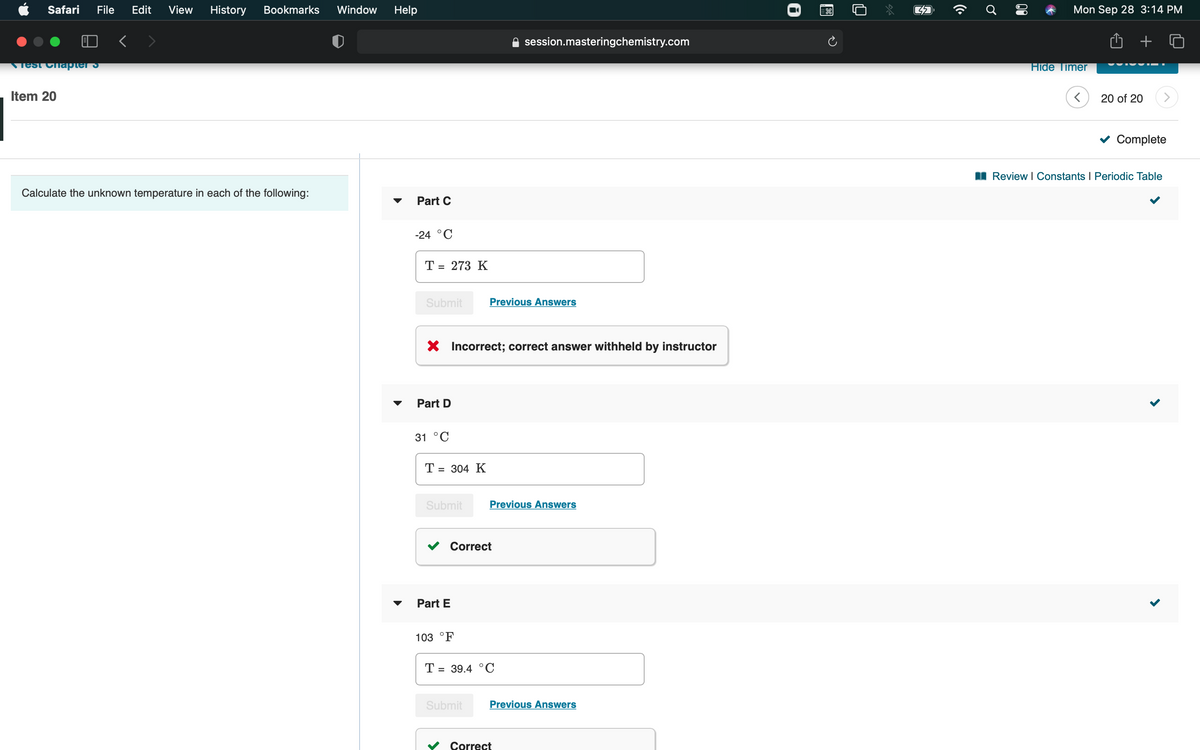
Need help on part C
Transcribed Image Text:Safari
File
Edit
View
History
Bookmarks
Window
Help
Mon Sep 28 3:14 PM
session.masteringchemistry.com
Test Chapter S
Hide Timer
Item 20
20 of 20
v Complete
II Review I Constants I Periodic Table
Calculate the unknown temperature
each
the following:
Part C
-24 °C
T = 273 K
%3D
Submit
Previous Answers
X Incorrect; correct answer withheld by instructor
Part D
31 °C
Т- 304 К
%3D
Submit
Previous Answers
Correct
Part E
103 °F
T = 39.4 °C
%3D
Submit
Previous Answers
Correct
00
Expert Solution
Introduction
The temperature scale is used to measure degree of hotness or coldness of a body (or system). The SI unit of temperature is Kelvin (K).
Given data:
The temperature is –24oC.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning