Part C °C? Air is composed mostly of N2 molecules, so you may assume that i kg. What is the rms speed vo of molecules in air at -26 has molecules of average mass 28.0 × 1.661 × 10-27 kg = 4.65 × 10° Express your answer in meters per second, to the nearest integer. ΑΣφ ? vo = |285 m/s Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining w consider a rigid dumbbell with two masses, each of mass m, spaced a distance d apart. Part D V dumbbell is lined up on the z axis and is in equilibrium at temperature T. Find 1/(w%)avg, the rms angular speed of the dumbbell about a single axis (taken to be the x axis), assuming that the
Part C °C? Air is composed mostly of N2 molecules, so you may assume that i kg. What is the rms speed vo of molecules in air at -26 has molecules of average mass 28.0 × 1.661 × 10-27 kg = 4.65 × 10° Express your answer in meters per second, to the nearest integer. ΑΣφ ? vo = |285 m/s Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining w consider a rigid dumbbell with two masses, each of mass m, spaced a distance d apart. Part D V dumbbell is lined up on the z axis and is in equilibrium at temperature T. Find 1/(w%)avg, the rms angular speed of the dumbbell about a single axis (taken to be the x axis), assuming that the
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter16: Temperature And The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 74P: A cylinder that has a 40.0-cm radius and is 50.0 cm deep is filled with air at 20.0C and 1.00 atm...
Related questions
Question
Need help with parts C, D, and E pls
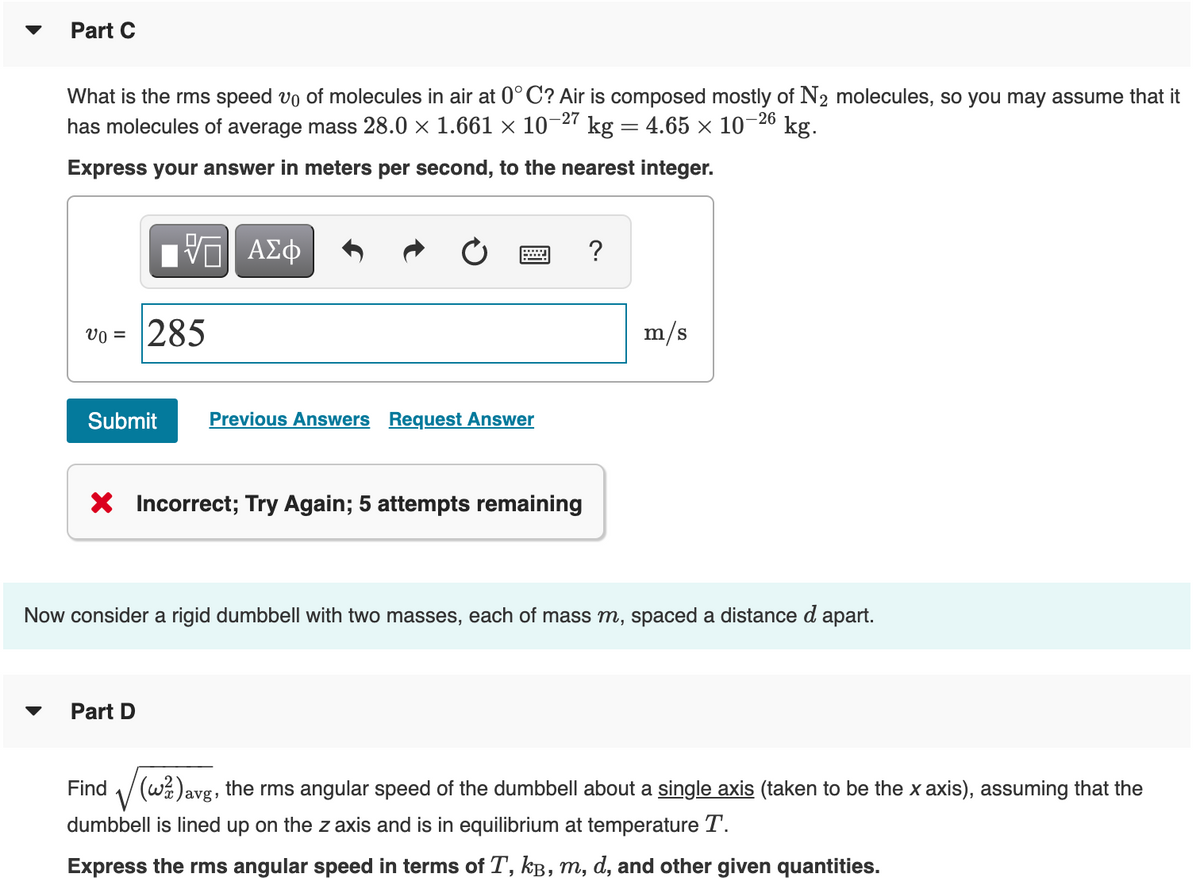
Transcribed Image Text:Part C
What is the rms speed vo of molecules in air at 0° C? Air is composed mostly of N2 molecules, so you may assume that it
has molecules of average mass 28.0 × 1.661 × 10-27 kg = 4.65 × 10¬2° kg.
Express your answer in meters per second, to the nearest integer.
ηνα ΑΣφ
?
Vo =
285
m/s
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
Now consider a rigid dumbbell with two masses, each of mass m, spaced a distance d apart.
Part D
Find
1/(w%)avg, the rms angular speed of the dumbbell about a single axis (taken to be the x axis), assuming that the
dumbbell is lined up on the z axis and is in equilibrium at temperature T.
Express the rms angular speed in terms of T, kB, m, d, and other given quantities.
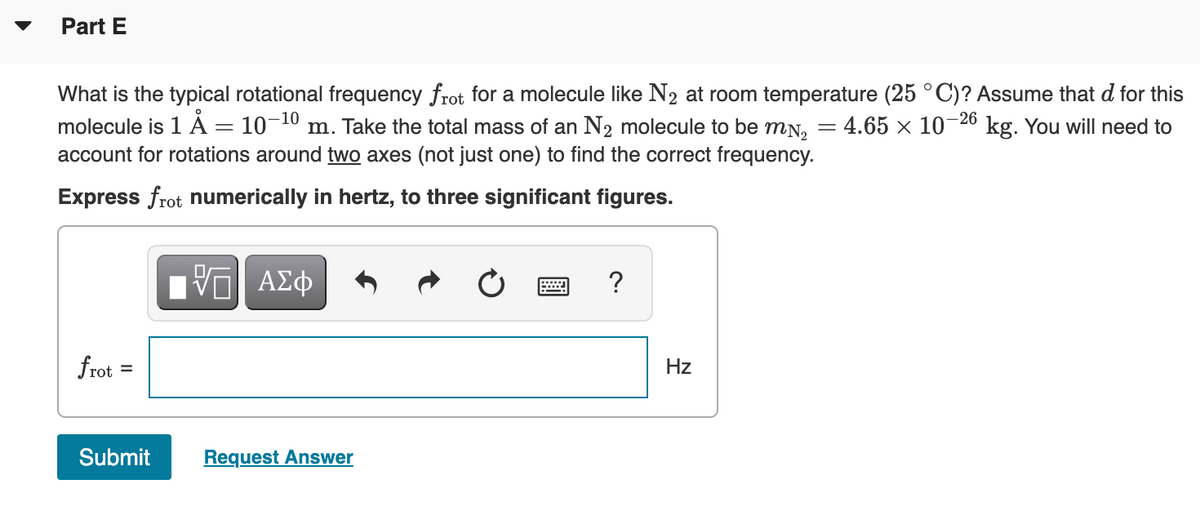
Transcribed Image Text:Part E
What is the typical rotational frequency frot for a molecule like N2 at room temperature (25 °C)? Assume that d for this
molecule is 1 A = 10¬10 m. Take the total mass of an N2 molecule to be mN, = 4.65 × 10¬26 kg. You will need to
account for rotations around two axes (not just one) to find the correct frequency.
Express frot numerically in hertz, to three significant figures.
?
frot
Hz
%D
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning