Part C Some hydrogen gas is enclosed within a chamber being held at 200° C with a volume of 0.0250 m³. The chamber is fitted with a movable piston. Initially, the pressure in the gas is 1.50 x 106 Pa (14.8 atm). The piston is slowly extracted until the pressure in the gas falls to 0.950 x 106 Pa. What is the final volume V₂ of the container? Assume that no gas escapes and that the temperature remains at 200°C. Enter your answer numerically in cubic meters. ▸ View Available Hint(s)
Part C Some hydrogen gas is enclosed within a chamber being held at 200° C with a volume of 0.0250 m³. The chamber is fitted with a movable piston. Initially, the pressure in the gas is 1.50 x 106 Pa (14.8 atm). The piston is slowly extracted until the pressure in the gas falls to 0.950 x 106 Pa. What is the final volume V₂ of the container? Assume that no gas escapes and that the temperature remains at 200°C. Enter your answer numerically in cubic meters. ▸ View Available Hint(s)
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter13: Temperature, Kinetic Theory, And The Gas Laws
Section: Chapter Questions
Problem 24PE: Suppose a gasfilled incandescent light bulb is manufactured so that the gas inside the bulb is at...
Related questions
Question
part c
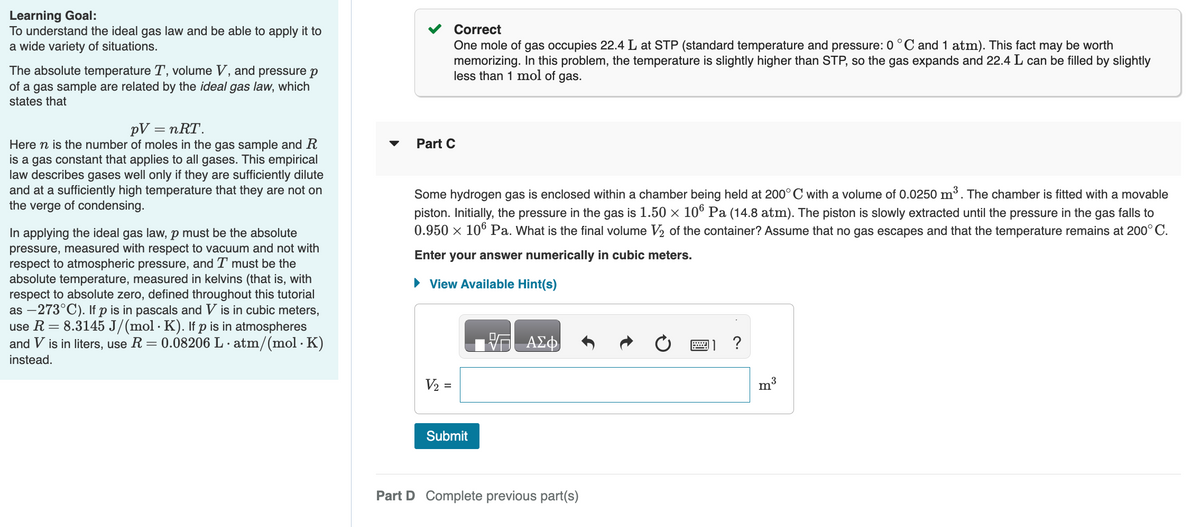
Transcribed Image Text:Learning Goal:
To understand the ideal gas law and be able to apply it to
a wide variety of situations.
The absolute temperature T, volume V, and pressure p
of a gas sample are related by the ideal gas law, which
states that
pV = nᎡᎢ .
Here n is the number of moles in the gas sample and R
is a gas constant that applies to all gases. This empirical
law describes gases well only if they are sufficiently dilute
and at a sufficiently high temperature that they are not on
the verge of condensing.
In applying the ideal gas law, p must be the absolute
pressure, measured with respect to vacuum and not with
respect to atmospheric pressure, and I must be the
absolute temperature, measured in kelvins (that is, with
respect to absolute zero, defined throughout this tutorial
as -273°C). If p is in pascals and V is in cubic meters,
use R = 8.3145 J/(mol · K). If p is in atmospheres
and V is in liters, use R = 0.08206 L.atm/(mol. K)
.
instead.
Correct
One mole of gas occupies 22.4 L at STP (standard temperature and pressure: 0 °C and 1 atm). This fact may be worth
memorizing. In this problem, the temperature is slightly higher than STP, so the gas expands and 22.4 L can be filled by slightly
less than 1 mol of gas.
Part C
Some hydrogen gas is enclosed within a chamber being held at 200° C with a volume of 0.0250 m³. The chamber is fitted with a movable
piston. Initially, the pressure in the gas is 1.50 × 106 Pa (14.8 atm). The piston is slowly extracted until the pressure in the gas falls to
0.950 × 106 Pa. What is the final volume V₂ of the container? Assume that no gas escapes and that the temperature remains at 200°C.
Enter your answer numerically in cubic meters.
► View Available Hint(s)
V₂ =
Submit
ΑΣΦ
Part D Complete previous part(s)
?
3
m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning