Part C What isotope has 13 protons and 14 neutrons? Enter the name of the element followed by a hyphen and the mass number (e.g., uranium-234). • View Available Hint(s) Part D Which element does X represent in the following expression: X? 15 Enter the chemical symbol of the element. • View Available Hint(s) X is the element symbol
Part C What isotope has 13 protons and 14 neutrons? Enter the name of the element followed by a hyphen and the mass number (e.g., uranium-234). • View Available Hint(s) Part D Which element does X represent in the following expression: X? 15 Enter the chemical symbol of the element. • View Available Hint(s) X is the element symbol
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter2: Atoms
Section: Chapter Questions
Problem 2.13P
Related questions
Question
Please answer 11 Part A, C, and D
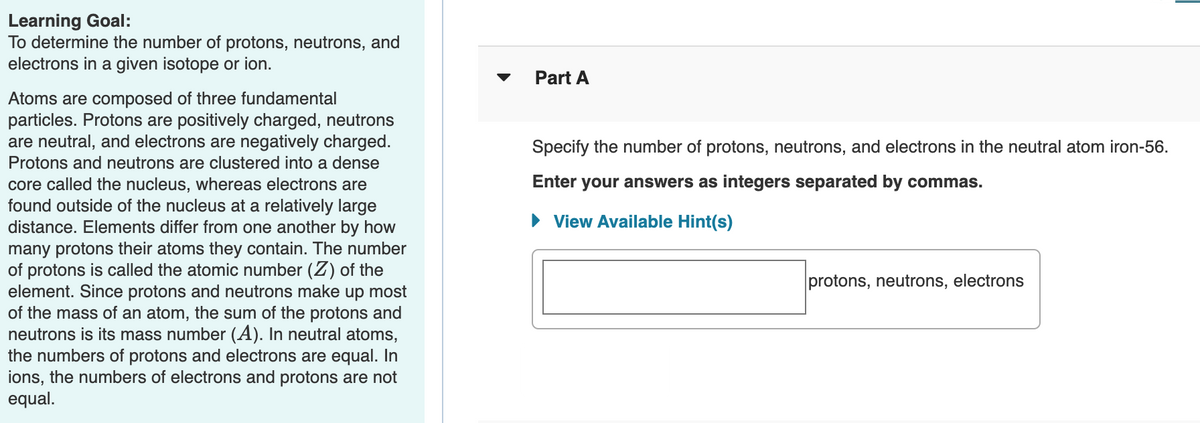
Transcribed Image Text:Learning Goal:
To determine the number of protons, neutrons, and
electrons in a given isotope or ion.
▼
Part A
Atoms are composed of three fundamental
particles. Protons are positively charged, neutrons
are neutral, and electrons are negatively charged.
Protons and neutrons are clustered into a dense
core called the nucleus, whereas electrons are
found outside of the nucleus at a relatively large
distance. Elements differ from one another by how
many protons their atoms they contain. The number
of protons is called the atomic number (Z) of the
element. Since protons and neutrons make up most
of the mass of an atom, the sum of the protons and
neutrons is its mass number (A). In neutral atoms,
the numbers of protons and electrons are equal. In
ions, the numbers of electrons and protons are not
equal.
Specify the number of protons, neutrons, and electrons in the neutral atom iron-56.
Enter your answers as integers separated by commas.
• View Available Hint(s)
protons, neutrons, electrons
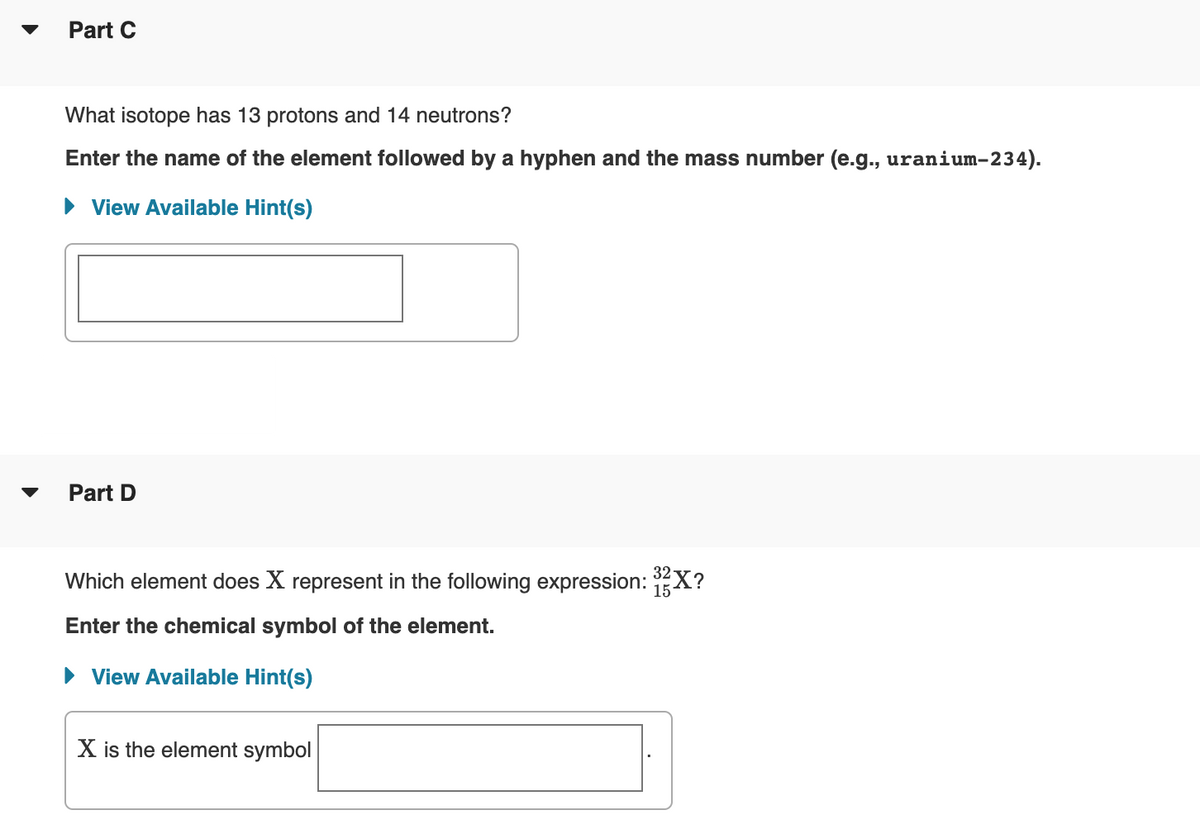
Transcribed Image Text:Part C
What isotope has 13 protons and 14 neutrons?
Enter the name of the element followed by a hyphen and the mass number (e.g., uranium-234).
• View Available Hint(s)
Part D
Which element does X represent in the following expression: X?
32
15-
Enter the chemical symbol of the element.
• View Available Hint(s)
X is the element symbol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
