Pb(CIO4)2 + 2 Kcı → PbCl2 + 2 KCIOA A chemist mixes dilute solutions of lead (II) perchlorate and potassium chloride to precipitate lead (II) chloride (Ksp = 1.7 x 105). 4. Which of the following is the net ionic equation for the experiment described above? (A) (B) (C) (D) 2 CI' + Pb*2 → PBCI2(s) 2 K*1 + ClO.? → K½CIO«(s) 2 CIO,1 + Pb*2 → PbCl2(s) + 4 O2(g) 4 CI + Pb*4 → PBCI4(s)
Pb(CIO4)2 + 2 Kcı → PbCl2 + 2 KCIOA A chemist mixes dilute solutions of lead (II) perchlorate and potassium chloride to precipitate lead (II) chloride (Ksp = 1.7 x 105). 4. Which of the following is the net ionic equation for the experiment described above? (A) (B) (C) (D) 2 CI' + Pb*2 → PBCI2(s) 2 K*1 + ClO.? → K½CIO«(s) 2 CIO,1 + Pb*2 → PbCl2(s) + 4 O2(g) 4 CI + Pb*4 → PBCI4(s)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 60QAP: Gold metal will dissolve only in aqua regia, a mixture of concentrated hydrochloric acid and...
Related questions
Question
Transcribed Image Text:Pb(CIO4)2 + 2 KCI → PbCl2 + 2 KCIO4
A chemist mixes dilute solutions of lead (II) perchlorate and potassium chloride to precipitate
lead (II) chloride (Ksp = 1.7 x 105).
4. Which of the following is the net ionic equation for the experiment described above?
(A)
(B)
(C)
(D)
2 CI' + Pb*2 → PbCl2(s)
2 K*1 + ClO, ? → K2CIOA(s)
2 ClO,1 + Pb+2 → PbCl2(s) + 4 02(g)
4 CI + Pb*4 → PbCla(s)
5. Within a factor of 10, what is the approximate molar solubility of PbCl2 in a solution of 0.20 M CI1
ions?
1.6 x 102 M
(A)
(B)
(C)
(D)
1.7 x 10$ M
5.0 x 103 M
4.0 x 10-4 M
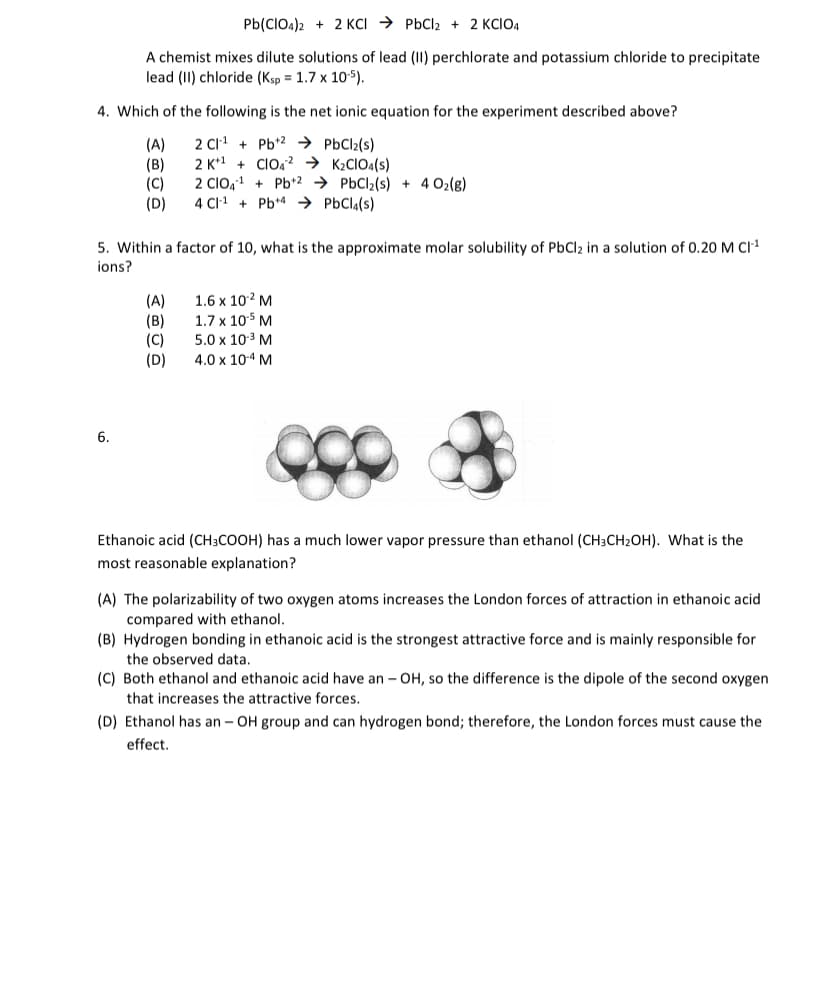
6.
Ethanoic acid (CH3COOH) has a much lower vapor pressure than ethanol (CH3CH2OH). What is the
most reasonable explanation?
(A) The polarizability of two oxygen atoms increases the London forces of attraction in ethanoic acid
compared with ethanol.
(B) Hydrogen bonding in ethanoic acid is the strongest attractive force and is mainly responsible for
the observed data.
(C) Both ethanol and ethanoic acid have an - OH, so the difference is the dipole of the second oxygen
that increases the attractive forces.
(D) Ethanol has an – OH group and can hydrogen bond; therefore, the London forces must cause the
effect.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning