Peptides are a linear (non branched) polymer made from amino acid residues. A protein consists of one or more large peptides and has a specific biological function. In order to best understand how proteins function, you first learned about their building blocks: amino acids. At pH=7, the predominant forms of amino acids contain a quaternary ammonium group and a carboxylate functional group. ● When carboxylate ions are in an aqueous solution, the carboxylate ion (base form) and carboxylic acid (acid form) conjugates are both present because they are in equilibrium. Likewise, when quaternary ammonium compounds are in an aqueous solution, the quaternary ammonium ion (acid form) and amine (base form) conjugates are both present. We can predict whether the acid form or the base form of these functional groups is predominant using the implications of the the Henderson-Hasselbalch relation; we compare the pH of the solution to the pK₂ of the group. 2 to 5 i) What is the pKa range of the carboxyl groups in amino acids? ii) What is the approximate pK₁ of the quaternary ammonium group in amino acids? ~9.5
Peptides are a linear (non branched) polymer made from amino acid residues. A protein consists of one or more large peptides and has a specific biological function. In order to best understand how proteins function, you first learned about their building blocks: amino acids. At pH=7, the predominant forms of amino acids contain a quaternary ammonium group and a carboxylate functional group. ● When carboxylate ions are in an aqueous solution, the carboxylate ion (base form) and carboxylic acid (acid form) conjugates are both present because they are in equilibrium. Likewise, when quaternary ammonium compounds are in an aqueous solution, the quaternary ammonium ion (acid form) and amine (base form) conjugates are both present. We can predict whether the acid form or the base form of these functional groups is predominant using the implications of the the Henderson-Hasselbalch relation; we compare the pH of the solution to the pK₂ of the group. 2 to 5 i) What is the pKa range of the carboxyl groups in amino acids? ii) What is the approximate pK₁ of the quaternary ammonium group in amino acids? ~9.5
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter15: Equilibria Of Other Reaction Classes
Section: Chapter Questions
Problem 82E: The simplest amino acid is glycine, H2NCH2CO2H. The common feature of amino acids is that they...
Related questions
Question
100%
I don't get it at all about part 1 and part 2. Can you help me why it was 2 to 5 for part 1? Can you help me why it was ~9.5? Can you help me to explain to me, please? I struggled.
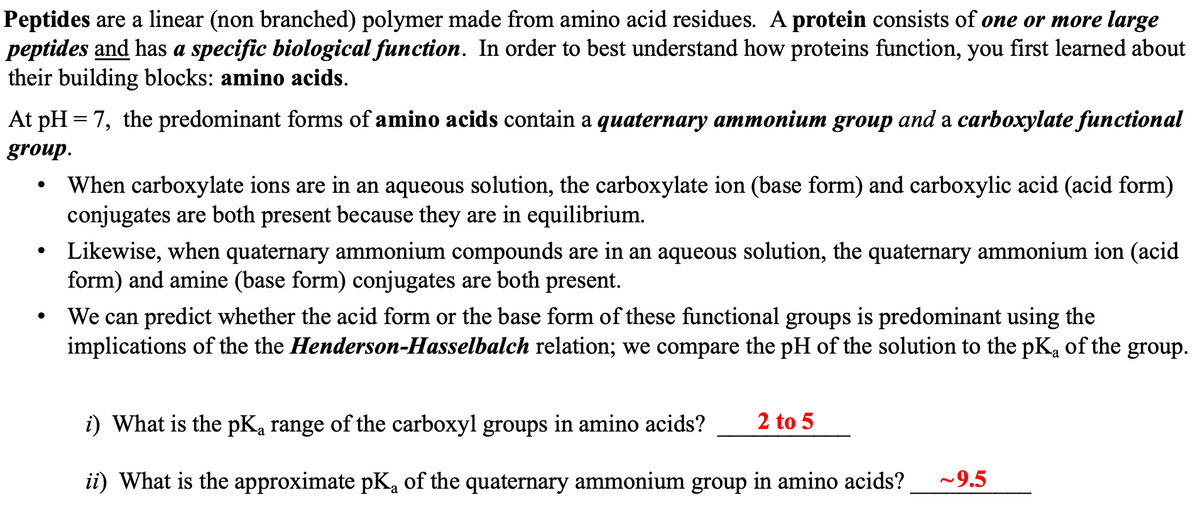
Transcribed Image Text:Peptides are a linear (non branched) polymer made from amino acid residues. A protein consists of one or more large
peptides and has a specific biological function. In order to best understand how proteins function, you first learned about
their building blocks: amino acids.
At pH=7, the predominant forms of amino acids contain a quaternary ammonium group and a carboxylate functional
group.
●
When carboxylate ions are in an aqueous solution, the carboxylate ion (base form) and carboxylic acid (acid form)
conjugates are both present because they are in equilibrium.
Likewise, when quaternary ammonium compounds are in an aqueous solution, the quaternary ammonium ion (acid
form) and amine (base form) conjugates are both present.
We can predict whether the acid form or the base form of these functional groups is predominant using the
implications of the the Henderson-Hasselbalch relation; we compare the pH of the solution to the pKa of the group.
i) What is the pKa range of the carboxyl groups in amino acids? 2 to 5
ii) What is the approximate pK₁ of the quaternary ammonium group in amino acids? ~9.5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
