Chapter14: Oxidation And Reduction
Section: Chapter Questions
Problem 3SC: Closely examine Figure 14.1 and explain why no reaction occurs in part d. Because copper ions have a...
Related questions
Question
PLEASE EXPLAIN THE PHOTO
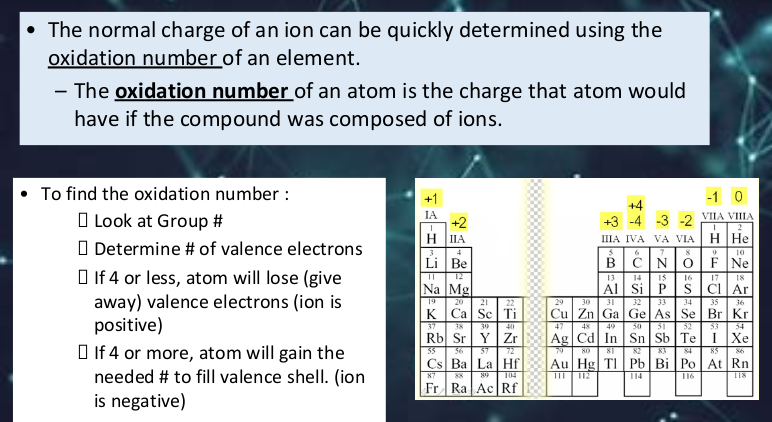
Transcribed Image Text:• The normal charge of an ion can be quickly determined using the
oxidation number of an element.
- The oxidation number of an atom is the charge that atom would
have if the compound was composed of ions.
To find the oxidation number :
+1
-1 0
+4
+3 -4 -3 -2 VIA VIILA
H He
O Look at Group #
I Determine # of valence electrons
O If 4 or less, atom will lose (give
away) valence electrons (ion is
positive)
IA
+2
H IIA
IIIA IVA VA VIA
6
BCN
3
4
10
Li Be
F Ne
11
12
13
14
IS
16
17
18
Na Mg
Al Si P
ciAr
19
20
21
22
29
30
31
32
33
34
35
36
K Ca Sc | Ti
Cu Zn Ga Ge As Se Br Kr
31
32
53
Ag Cd In Sn Sb Te
37
38
39
40
47
48
49
50
54
Rb Sr Y|Zr
I Xe
O If 4 or more, atom will gain the
needed # to fill valence shell. (ion
is negative)
55
56
57
72
80 8T
Au Hg| TI Pb Bi Po At Rn
79
82
83
84
85
Cs Ba La Hf
87
89
104
112
114
116
118
Fr Ra Ac Rf
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning