Chapter6: The States Of Matter
Section: Chapter Questions
Problem 6.9E
Related questions
Question
please help find average and standard deviation
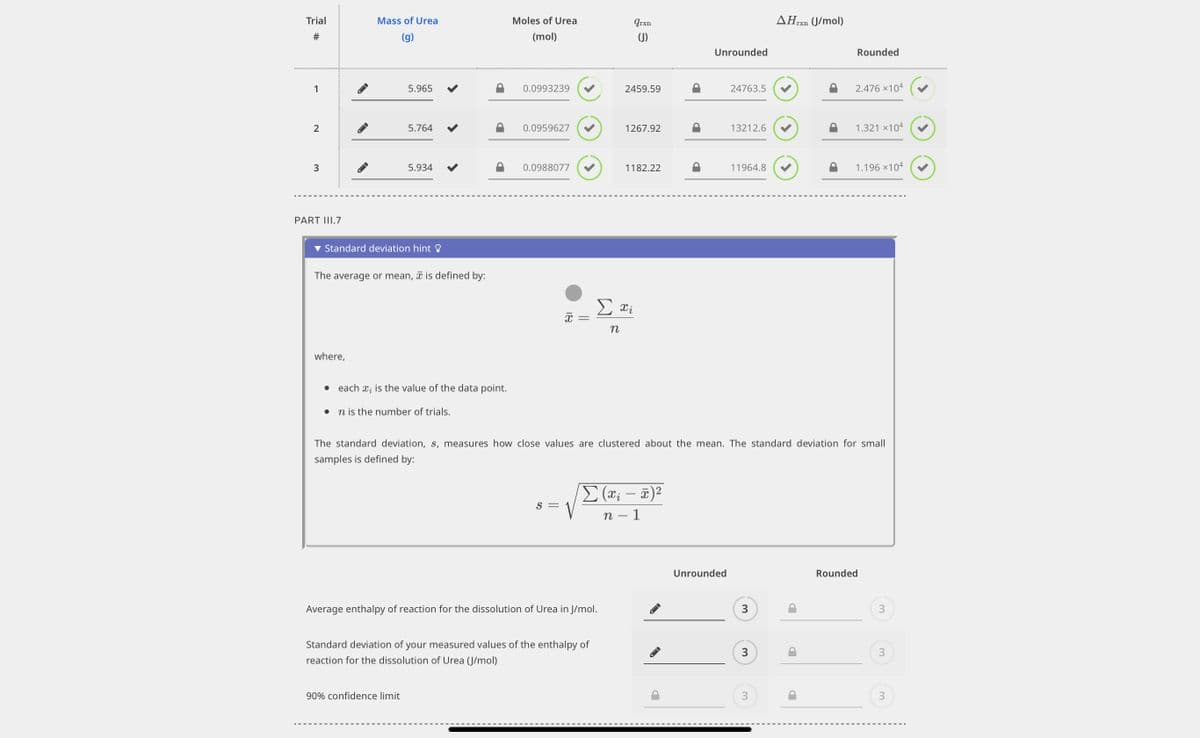
Transcribed Image Text:Trial
#
1
2
3
PART III.7
Mass of Urea
(g)
where,
5.965
5.764
Standard deviation hint
5.934
The average or mean, is defined by:
• each ₂ is the value of the data point.
n is the number of trials.
90% confidence limit
Moles of Urea
(mol)
0.0993239
0.0959627
0.0988077
18
S =
Average enthalpy of reaction for the dissolution of urea in J/mol.
Standard deviation of your measured values of the enthalpy of
reaction for the dissolution of Urea (J/mol)
qrxn
(J)
2459.59
1267.92
1182.22
Σαΐ
n
Σ (x₁ - x)²
n - 1
Unrounded
24763.5
Unrounded
13212.6
11964.8
The standard deviation, s, measures how close values are clustered about the mean. The standard deviation for small
samples is defined by:
3
3
AHrxn
3
(J/mol)
P
Rounded
2.476 ×104
1.321 ×104
1.196 ×104
Rounded
3
3
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 13 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you







Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning