Please solve the average molarity of the calcium hydroxide solution to determine the concentration (M) Ca2+ and OH-: [Ca2+] = ? [ОН-] —? Additionally, determine the Ksp for calcium hydroxide. THANK YOU!
Please solve the average molarity of the calcium hydroxide solution to determine the concentration (M) Ca2+ and OH-: [Ca2+] = ? [ОН-] —? Additionally, determine the Ksp for calcium hydroxide. THANK YOU!
Chapter9: Acids, Bases, And Salts
Section: Chapter Questions
Problem 9.107E
Related questions
Question
100%
Please see attached photos and answer accordingly. Thank you.
![Please solve the average molarity
of the calcium hydroxide solution
to determine the concentration
(М) Са2+ and OH-:
[Ca2+] = ?
[OH-] = ?
Additionally, determine the Ksp
for calcium hydroxide. THANK
YOU!](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd7e141d7-49c5-44f7-9f3c-7e5d88d63be5%2F4b36a6c8-2ddd-4c50-a76c-b9653f852e0d%2Fxpj0rn_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Please solve the average molarity
of the calcium hydroxide solution
to determine the concentration
(М) Са2+ and OH-:
[Ca2+] = ?
[OH-] = ?
Additionally, determine the Ksp
for calcium hydroxide. THANK
YOU!
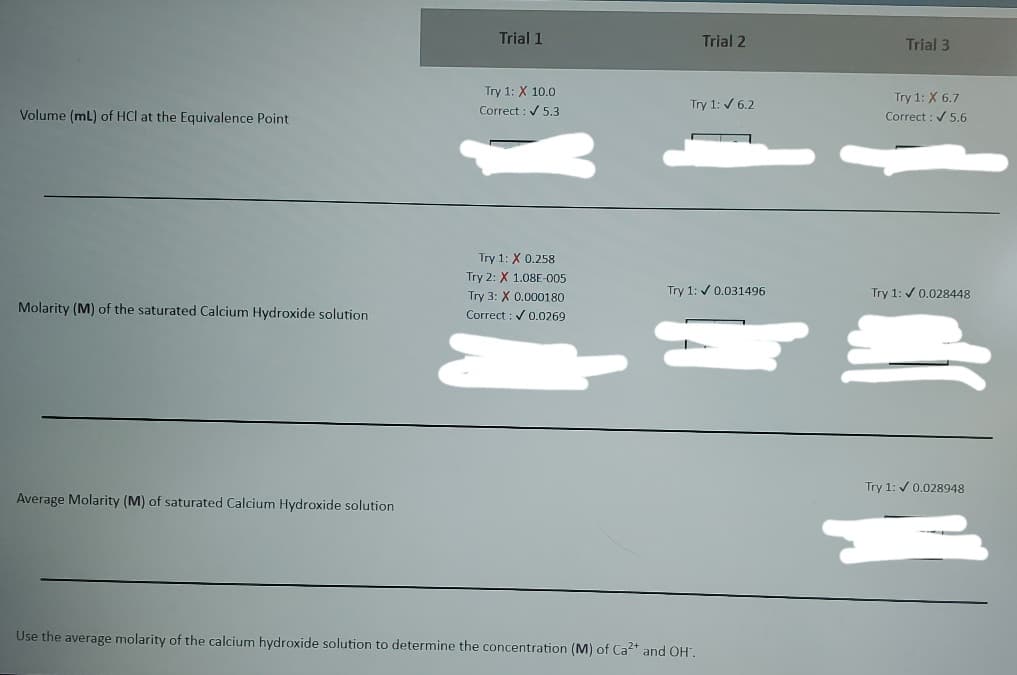
Transcribed Image Text:Trial 1
Trial 2
Trial 3
Try 1: X 10.0
Correct : V 5,3
Try 1: X 6.7
Correct : / 5.6
Try 1: / 6.2
Volume (mL) of HCl at the Equivalence Point
Try 1: X 0.258
Try 2: X 1.08E-005
Try 3: X 0.000180
Try 1: v 0.031496
Try 1: / 0.028448
Molarity (M) of the saturated Calcium Hydroxide solution
Correct : V 0.0269
Try 1: / 0.028948
Average Molarity (M) of saturated Calcium Hydroxide solution
Use the average molarity of the calcium hydroxide solution to determine the concentration (M) of Ca2+ and OH".
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning