Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.35QAP
Related questions
Question
100%
please do this typewritten so I will upvote and skip this if you already did this. the big number on the right is only for numbering. please do not mind that. thank you
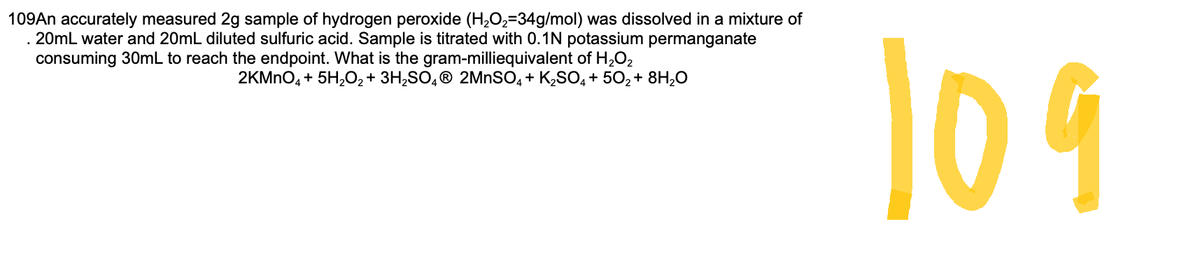
Transcribed Image Text:109An accurately measured 2g sample of hydrogen peroxide (H₂O₂-34g/mol) was dissolved in a mixture of
. 20mL water and 20mL diluted sulfuric acid. Sample is titrated with 0.1N potassium permanganate
consuming 30mL to reach the endpoint. What is the gram-milliequivalent of H₂O₂
2KMnO4 + 5H₂O₂ + 3H₂SO4Ⓡ 2MnSO4 + K₂SO4 +50₂ + 8H₂O
109
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning