POSITIVE ION (CATION) NEGATIVE ION (ANION) Activity 1. Positive Ions (Cations) Direction: Determine the number of electron, proton, and neutron in positive ions (cation). Write your answer inside the box on the space provided. 1. 2. 40 48 " Al3+ 20 Ca2+ 27 22 Number of Number of Protons" Number of Protons Protons Number of Number of Number of Neutrons Neutrons Neutrons Number of Number of Number of Electrons Electrons Electrons 23 24 : Mg* i Na+ 12 Number of Protons Number of Protons Number of Neutrons Number of Neutrons- Number of Electrons, Number of Electrons
POSITIVE ION (CATION) NEGATIVE ION (ANION) Activity 1. Positive Ions (Cations) Direction: Determine the number of electron, proton, and neutron in positive ions (cation). Write your answer inside the box on the space provided. 1. 2. 40 48 " Al3+ 20 Ca2+ 27 22 Number of Number of Protons" Number of Protons Protons Number of Number of Number of Neutrons Neutrons Neutrons Number of Number of Number of Electrons Electrons Electrons 23 24 : Mg* i Na+ 12 Number of Protons Number of Protons Number of Neutrons Number of Neutrons- Number of Electrons, Number of Electrons
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.14QAP
Related questions
Question
answer all
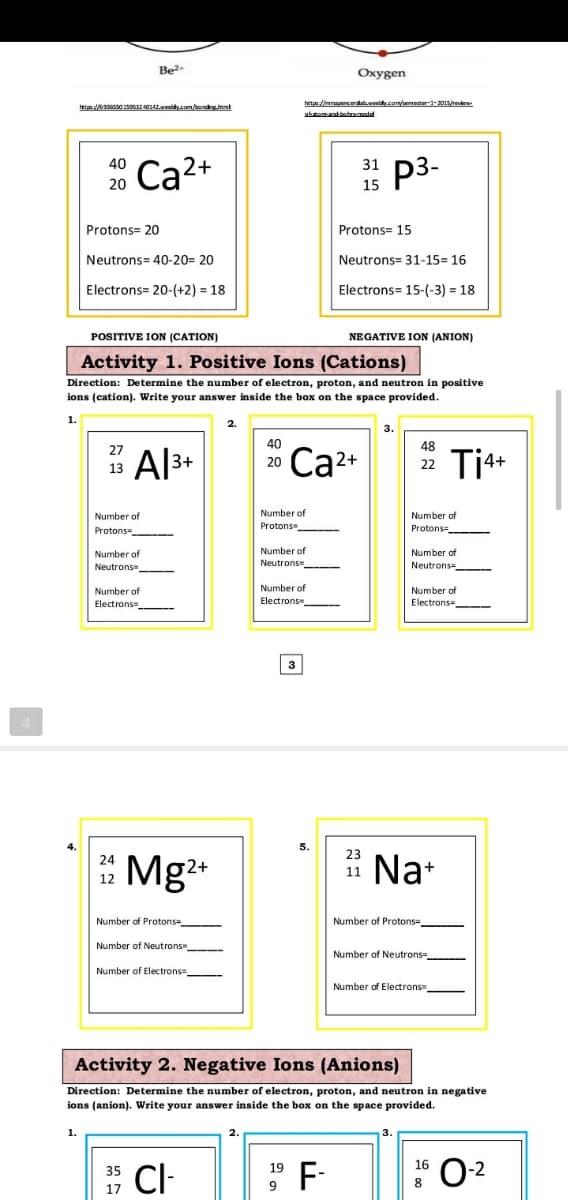
Transcribed Image Text:Be?
Oxygen
etpa: s0 19o532 40142.weethycom/bondinghtnl
hetp:/imrapercerdaweelyom/emeter-201revie
p3-
40
31
Ca2+
20
15
Protons= 20
Protons= 15
Neutrons= 40-20= 20
Neutrons= 31-15= 16
Electrons= 20-(+2) = 18
Electrons= 15-(-3) = 18
POSITIVE ION (CATION)
NEGATIVE ION (ANION)
Activity 1. Positive Ions (Cations)
Direction: Determine the number
I electron, proton, and neutron in positive
ions (cation). Write your answer inside the box on the space provided.
1.
3.
40
48
27
Al3+
Ca2+
Ti*+
20
22
13
Number of
Number of
Protons
Number of
Protons
Protons-
Number of
Number of
Number of
Neutrons
Neutrons=
Neutrons -
Number of
Electrons
Number of
Number of
Flectrons -
Electrons=
3
4.
5.
23
*
Mg2*
Na+
24
11
12
Number of Protons
Number of Protons=
Number of Neutrons
Number of Neutrons=
Number
Electrons=
Number of Electrons
Activity 2. Negative Ions (Anions)
Direction: Determine the number of electron, proton, and neutron in negative
ions (anion). Write your answer inside the box on the space provided.
1.
CI-
F-
0-2
35
19
16
8
17
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

