Potassium nitrate (KNO3) crystals were insoluble in water at room temperature. Heating the solution increases the solubility of the crystals in water. Briefly explain this phenomenon based on intermolecular forces of attraction of molecules.
Potassium nitrate (KNO3) crystals were insoluble in water at room temperature. Heating the solution increases the solubility of the crystals in water. Briefly explain this phenomenon based on intermolecular forces of attraction of molecules.
An Introduction to Physical Science
14th Edition
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Chapter13: Chemical Reactions
Section: Chapter Questions
Problem 14FIB
Related questions
Question
Potassium nitrate (KNO3) crystals were insoluble in water at room temperature. Heating the solution increases the solubility of the crystals in water.
Briefly explain this phenomenon based on intermolecular forces of attraction of molecules.
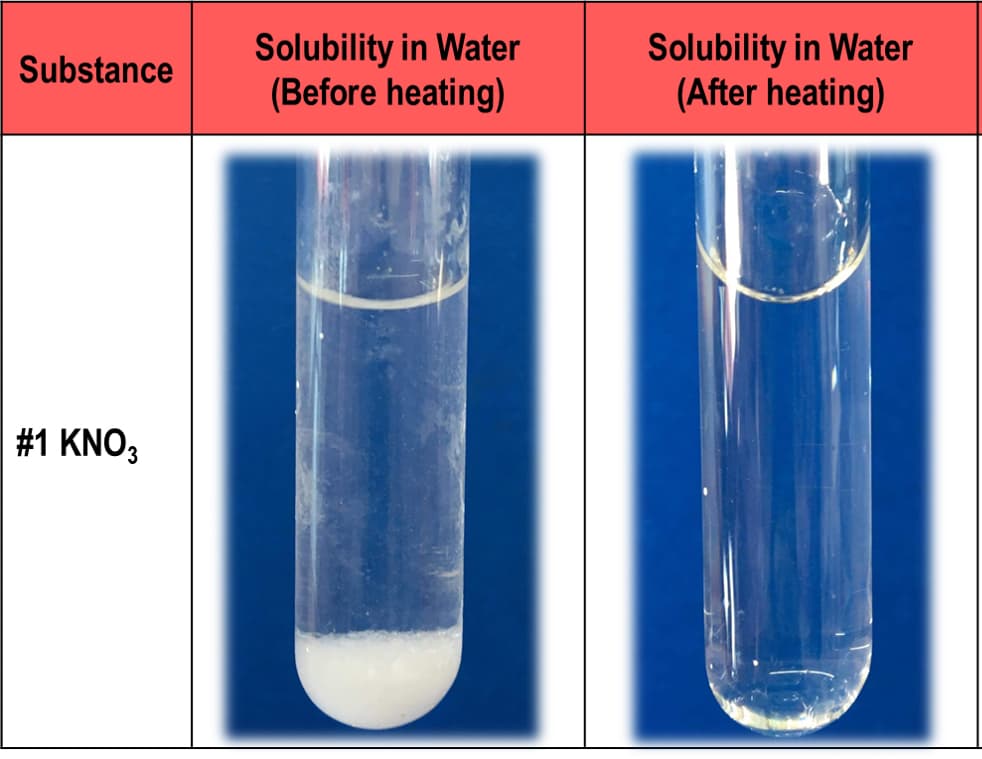
Transcribed Image Text:Solubility in Water
(Before heating)
Solubility in Water
(After heating)
Substance
#1 KNO3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning


An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning
