power output of a laser is measured by its wattage, the number of joules of energy it radiates per second (1 W = 1 J s). A 75.0-mW krypton laser produces a beam of blue light with a wavelength of 476.2 nm (4.762×10- m). The (a) Calculate the energy carried by each photon. (b) Calculate the number of photons emitted by the laser per second. (a) per photon (b) photons per second
power output of a laser is measured by its wattage, the number of joules of energy it radiates per second (1 W = 1 J s). A 75.0-mW krypton laser produces a beam of blue light with a wavelength of 476.2 nm (4.762×10- m). The (a) Calculate the energy carried by each photon. (b) Calculate the number of photons emitted by the laser per second. (a) per photon (b) photons per second
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter6: The Periodic Table And Atomic Structure
Section: Chapter Questions
Problem 6.17PAE: 6.17 The laser in most supermarket barcode scanners operates at a wavelength of 632.8 nm. What is...
Related questions
Question
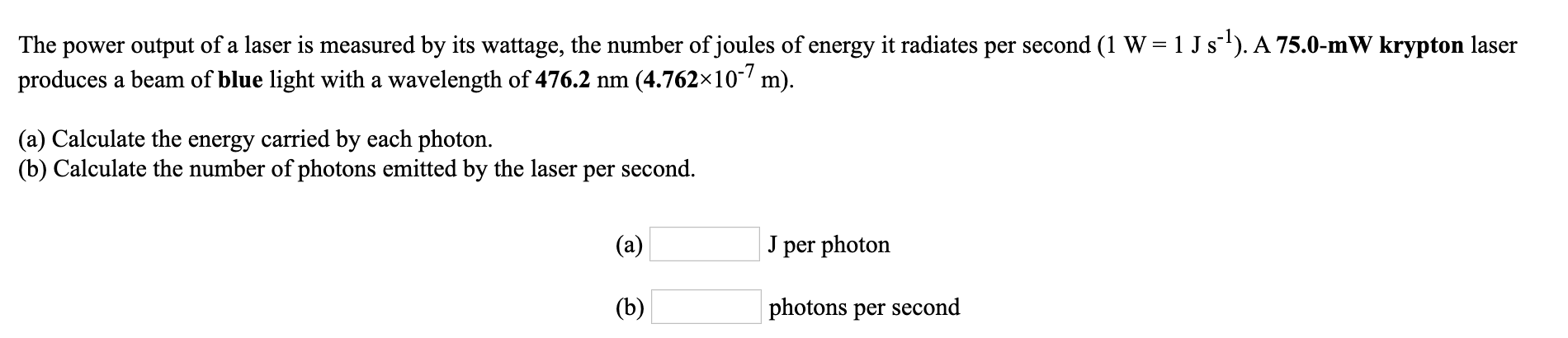
Transcribed Image Text:power output of a laser is measured by its wattage, the number of joules of energy it radiates per second (1 W = 1 J s). A 75.0-mW krypton laser
produces a beam of blue light with a wavelength of 476.2 nm (4.762×10- m).
The
(a) Calculate the energy carried by each photon.
(b) Calculate the number of photons emitted by the laser per second.
(a)
per photon
(b)
photons per second
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
