Predict the products and propose mechanisms for the reactions of ketones and aldehydes with oxidizing and reducing agents, amines, alcohols, and phosphorus ylides.
Predict the products and propose mechanisms for the reactions of
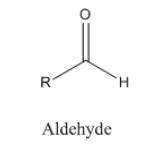
The general structure of aldehyde is shown below:
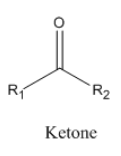
The general structure of ketone is given below.
Reaction of Aldehydes:
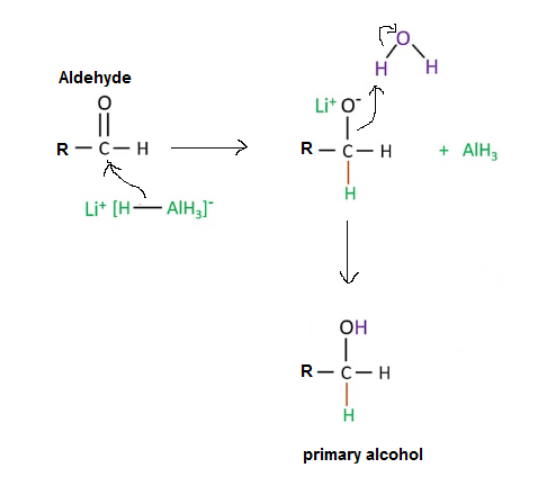
Aldehyde + reducing agent (LiAlH4) ----> Primary Alcohol
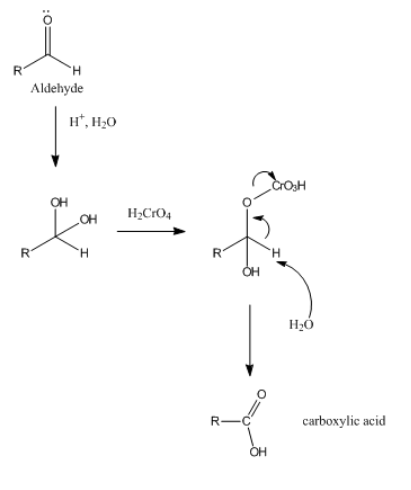
Aldehyde + Oxidizing agent ----> Carboxylic Acid
Aldehyde + Amine ----> Imine
Aldehyde + Alcohol ----> Hemiacetal
Aldehyde + phosphorous ylide ----> Alkene
Reaction of Ketones:
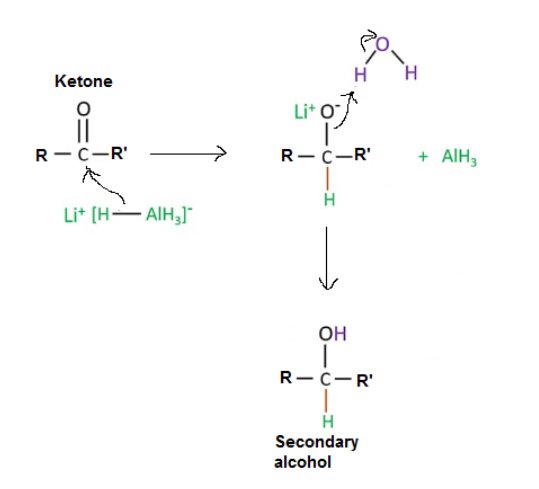
Ketone + reducing agent (LiAlH4) ----> Secondary Alcohol
Ketone+ Oxidizing agent ----> No reaction
Ketone + Amine ----> Imine
Ketone + Alcohol ----> Hemiketal
Ketone + phosphorous ylide ----> Alkene
1. Reaction of an aldehyde and ketone with reducing agent (LiAlH4) is shown below.
2. Reaction of Aldehyde and ketone with oxidizing agent is shown below.

Step by step
Solved in 7 steps with 11 images






