Predict what will be observed in each experiment below. experiment A student has two unopened 33 cL cans containing carbonated water. Can A has been stored in the garage (32 °C) and can B has been stored in the fridge (8 °C). The student opens one can at the time, both cans make a fizz. O A student sees tiny bubbles clinging to the inside of an unopened plastic bottle full of carbonated soft drink. The student squeezes the bottle. Can A will make a louder and stronger fizz than can B. O Can B will make a louder and stronger fizz than can A. predicted observation (choose one) O The fizz will be the same for both cans. There is not enough information to predict which can will make the louder fizz. O The bubbles will shrink, and some may vanish. O The bubbles will grow, and more may appear. O The bubbles won't change. O I need more information to predict what will happen to the bubbles.
Predict what will be observed in each experiment below. experiment A student has two unopened 33 cL cans containing carbonated water. Can A has been stored in the garage (32 °C) and can B has been stored in the fridge (8 °C). The student opens one can at the time, both cans make a fizz. O A student sees tiny bubbles clinging to the inside of an unopened plastic bottle full of carbonated soft drink. The student squeezes the bottle. Can A will make a louder and stronger fizz than can B. O Can B will make a louder and stronger fizz than can A. predicted observation (choose one) O The fizz will be the same for both cans. There is not enough information to predict which can will make the louder fizz. O The bubbles will shrink, and some may vanish. O The bubbles will grow, and more may appear. O The bubbles won't change. O I need more information to predict what will happen to the bubbles.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter6: Solutions And Colloids
Section: Chapter Questions
Problem 6.93P: 6-93 Two bottles of water are carbonated, with CO2 gas being added, under 2 atm pressure and then...
Related questions
Question
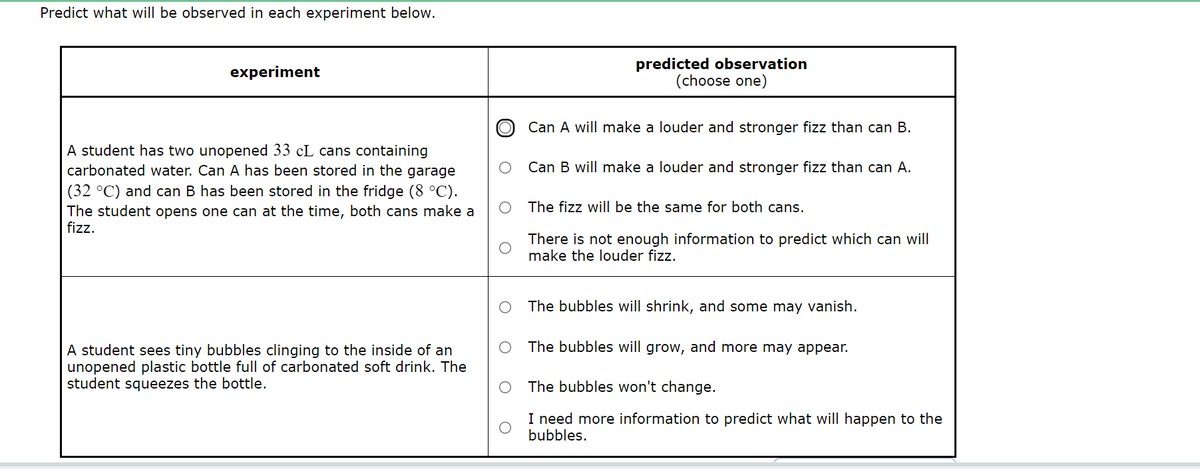
Transcribed Image Text:Predict what will be observed in each experiment below.
experiment
A student has two unopened 33 cL cans containing
carbonated water. Can A has been stored in the garage
(32 °C) and can B has been stored in the fridge (8 °C).
The student opens one can at the time, both cans make a
fizz.
O
A student sees tiny bubbles clinging to the inside of an
unopened plastic bottle full of carbonated soft drink. The
student squeezes the bottle.
Can A will make a louder and stronger fizz than can B.
O
Can B will make a louder and stronger fizz than can A.
The fizz will be the same for both cans.
There is not enough information to predict which can will
make the louder fizz.
O
predicted observation
(choose one)
O
The bubbles will shrink, and some may vanish.
The bubbles will grow, and more may appear.
The bubbles won't change.
O
I need more information to predict what will happen to the
bubbles.
Expert Solution
Step 1
1) During the process of carbonation co2 is released and amount of co2 released is directly proportional to temperature . We can see that temperature of can A is high so it will release more co2 as more heat is present in can A due to which solubility of co2 in Can A . cAn B have low temperature and hence less co2 will release .
so we can conclude that can A will make louder fizz than can B .
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
