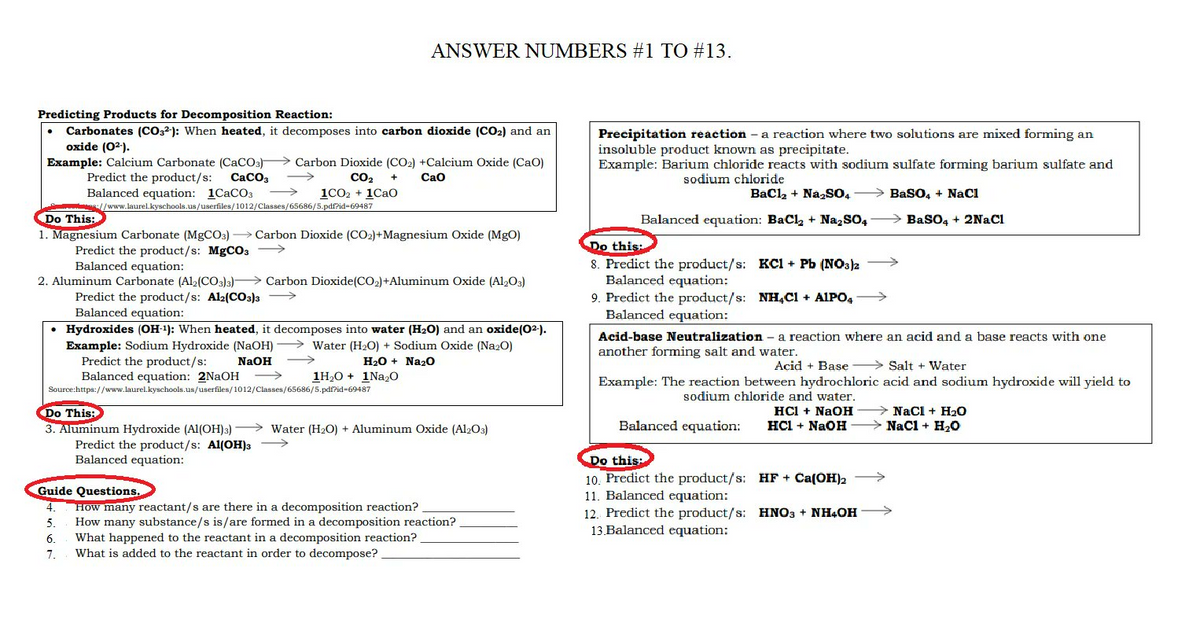
Predicting Products for Decomposition Reaction: Carbonates (CO2): When heated, it decomposes into carbon dioxide (CO₂) and an oxide (0²). Example: Calcium Carbonate (CaCO3) Carbon Dioxide (CO₂) +Calcium Oxide (Cao) Predict the product/s: CaCO3 → CO₂ + CaO Balanced equation: 1CaCO3 1CO₂ + 1CaO /www.laurel.kyschools.us/userfiles/1012/Classes/65686/5.pdf?id-69487 Do This: 1. Magnesium Carbonate (MgCO3)→ Carbon Dioxide (CO₂)+Magnesium Oxide (MgO) Predict the product/s: MgCO3 → Balanced equation: 2. Aluminum Carbonate (Al2(CO3)3) Carbon Dioxide (CO₂) +Aluminum Oxide (Al₂O3) Predict the product/s: Al2(CO3)3 → Balanced equation: • Hydroxides (OH-1): When heated, it decomposes into water (H₂O) and an oxide(02-). Example: Sodium Hydroxide (NaOH) Water (H₂O) + Sodium Oxide (Na₂O) H₂O + Na₂0 Predict the product/s: NaOH Balanced equation: 2NaOH →>> Source:https://www.laurel.kyschools.us/userfiles/1012/Classes/65686/5.pdf?id-69487 1H₂O+1Na₂O Do This 3. Aluminum Hydroxide (Al(OH)3)→ Water (H₂O) + Aluminum Oxide (Al2O3) Predict the product/s: Al(OH)3 →→ Balanced equation: Guide Questions. 4. How many reactant/s are there in a decomposition reaction? How many substance/s is/are formed in a decomposition reaction? 5. 6. What happened to the reactant in a decomposition reaction? 7. What is added to the reactant in order to decompose? Precipitation reaction - a reaction where two solutions are mixed forming an insoluble product known as precipitate. Example: Barium chloride reacts with sodium sulfate forming barium sulfate and sodium chloride BaCl₂ + Na₂SO4 →BaSO, + NaCl BaSO4 + 2NaCl Balanced equation: BaCl₂ + Na₂SO4 Do this: 8. Predict the product/s: KC1+ Pb (NO3)2 Balanced equation: 9. Predict the product/s: NH,C1 + AIPO4 → Balanced equation: Acid-base Neutralization - a reaction where an acid and a base reacts with one another forming salt and water. Acid + Base Salt + Water Example: The reaction between hydrochloric acid and sodium hydroxide will yield to sodium chloride and water. HCl + NaOH HCl + NaOH Balanced equation: Do this: 10. Predict the product/s: 11. Balanced equation: 12. Predict the product/s: 13.Balanced equation: NaCl + H₂O → NaCl + H₂O HF + Ca(OH)2 HNO3 + NH4OH →→→→
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps







