Predicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: acid base Ka K, name formula name formula -4 hydrocyanic acid HCN 4.9 x 10-10 methylamine CH;NH, | 4.4 × 10 -4 hypochlorous acid HC1O 3.0 x 10-8 ethylamine C,H;NH, 6.4 × 10* Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the solution that will have the next lowest pH, and so on. solution pH 0.1 M NACN choose one v 0.1 M C2H5NH3Br choose one v 0.1 М KCIO choose one v 0.1 M NaNO3 choose one v
Predicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: acid base Ka K, name formula name formula -4 hydrocyanic acid HCN 4.9 x 10-10 methylamine CH;NH, | 4.4 × 10 -4 hypochlorous acid HC1O 3.0 x 10-8 ethylamine C,H;NH, 6.4 × 10* Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the solution that will have the next lowest pH, and so on. solution pH 0.1 M NACN choose one v 0.1 M C2H5NH3Br choose one v 0.1 М KCIO choose one v 0.1 M NaNO3 choose one v
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.5QAP
Related questions
Question
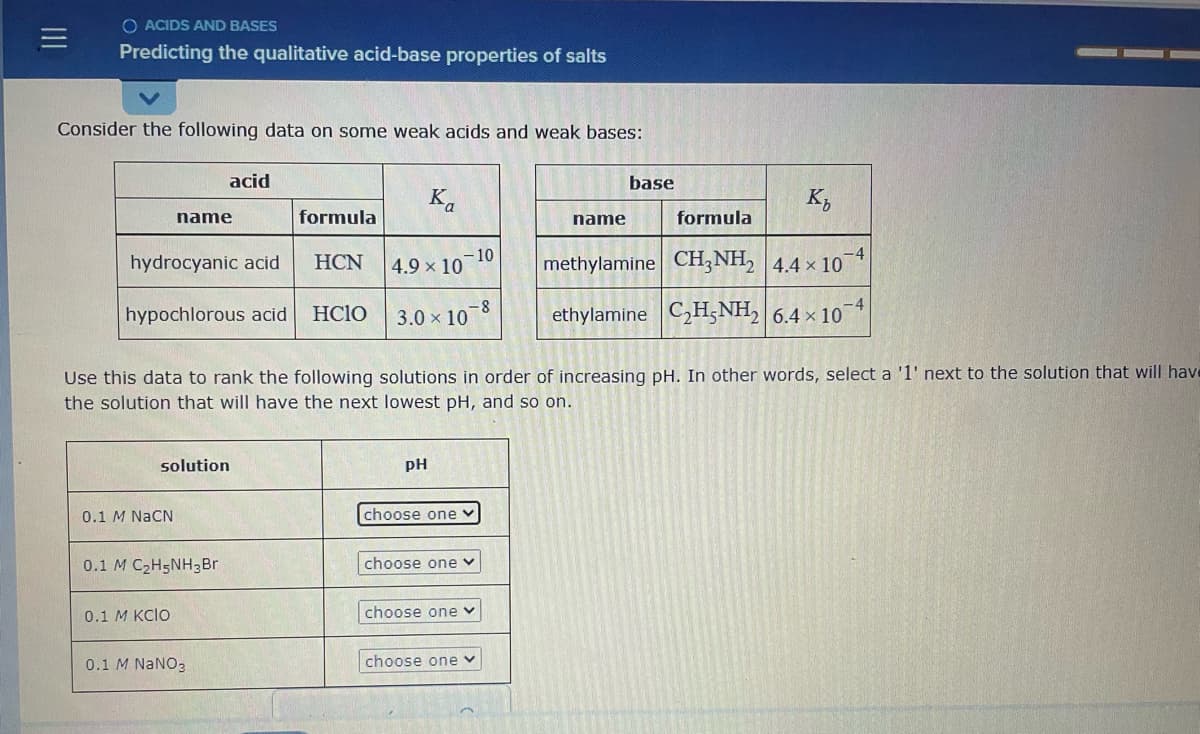
Transcribed Image Text:O ACIDS AND BASES
Predicting the qualitative acid-base properties of salts
Consider the following data on some weak acids and weak bases:
acid
base
Ka
K,
name
formula
name
formula
- 10
4.9 x 10
-4
hydrocyanic acid
HCN
methylamine CH;NH, 4.4 x 10
hypochlorous acid
HCIO
3.0 x 10-8
ethylamine C,H;NH, 6.4 × 104
Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have
the solution that will have the next lowest pH, and so on.
solution
pH
0.1 M NACN
choose one
0.1 M C2H5NH3Br
choose one v
0.1 М KCIO
choose one v
0.1 M NANO3
choose one v
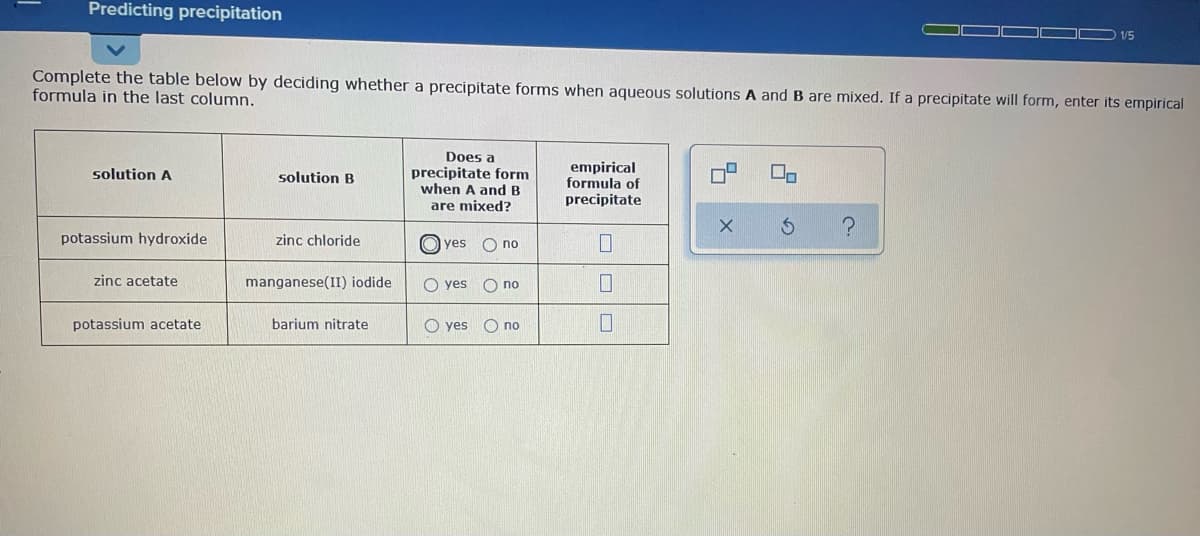
Transcribed Image Text:Predicting precipitation
1/5
Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical
formula in the last column.
Does a
precipitate form
when A and B
empirical
formula of
precipitate
solution A
solution B
are mixed?
potassium hydroxide
zinc chloride
O yes O no
zinc acetate
manganese(II) iodide
O yes O no
potassium acetate
barium nitrate
O yes
O no
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

