Problem 1: In statistical mechanics, the internal energy of an ideal gas is given by: N 2/3 2S U = U(S, V) = aNkB 3NKB e where a is a constant. 1- Show that the variation of the internal energy is given by: (s, ") as - Gu). 2 U).dV 3V dU = .dS - 3M 2- Using the fundamental relation of thermodynamic dU = T.ds – p.dV, show that the equation of state PV = nRT follows from the first expression of U.
Problem 1: In statistical mechanics, the internal energy of an ideal gas is given by: N 2/3 2S U = U(S, V) = aNkB 3NKB e where a is a constant. 1- Show that the variation of the internal energy is given by: (s, ") as - Gu). 2 U).dV 3V dU = .dS - 3M 2- Using the fundamental relation of thermodynamic dU = T.ds – p.dV, show that the equation of state PV = nRT follows from the first expression of U.
Related questions
Question
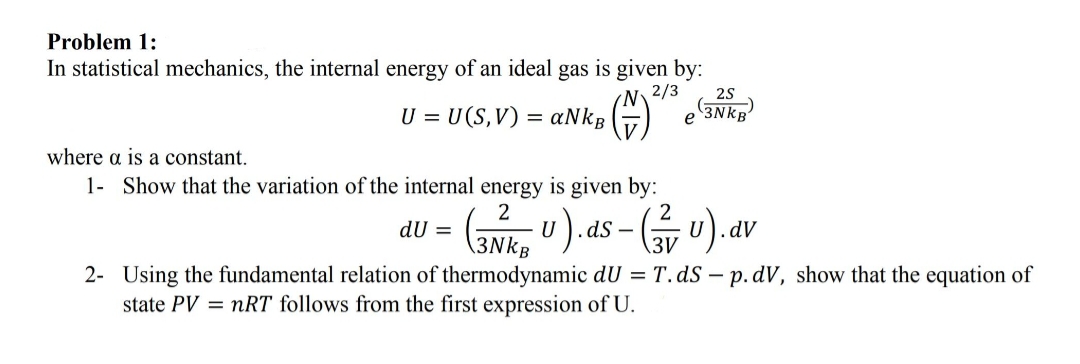
Transcribed Image Text:Problem 1:
In statistical mechanics, the internal energy of an ideal gas is given by:
N.
aNkB
2/3
(3NKB
U = U(S,V) =
е
where a is a constant.
1- Show that the variation of the internal energy is given by:
2
dS -
\3V
2
dU =
dV
\3NkB
2- Using the fundamental relation of thermodynamic dU = T.ds – p. dV, show that the equation of
state PV = nRT follows from the first expression of U.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps
