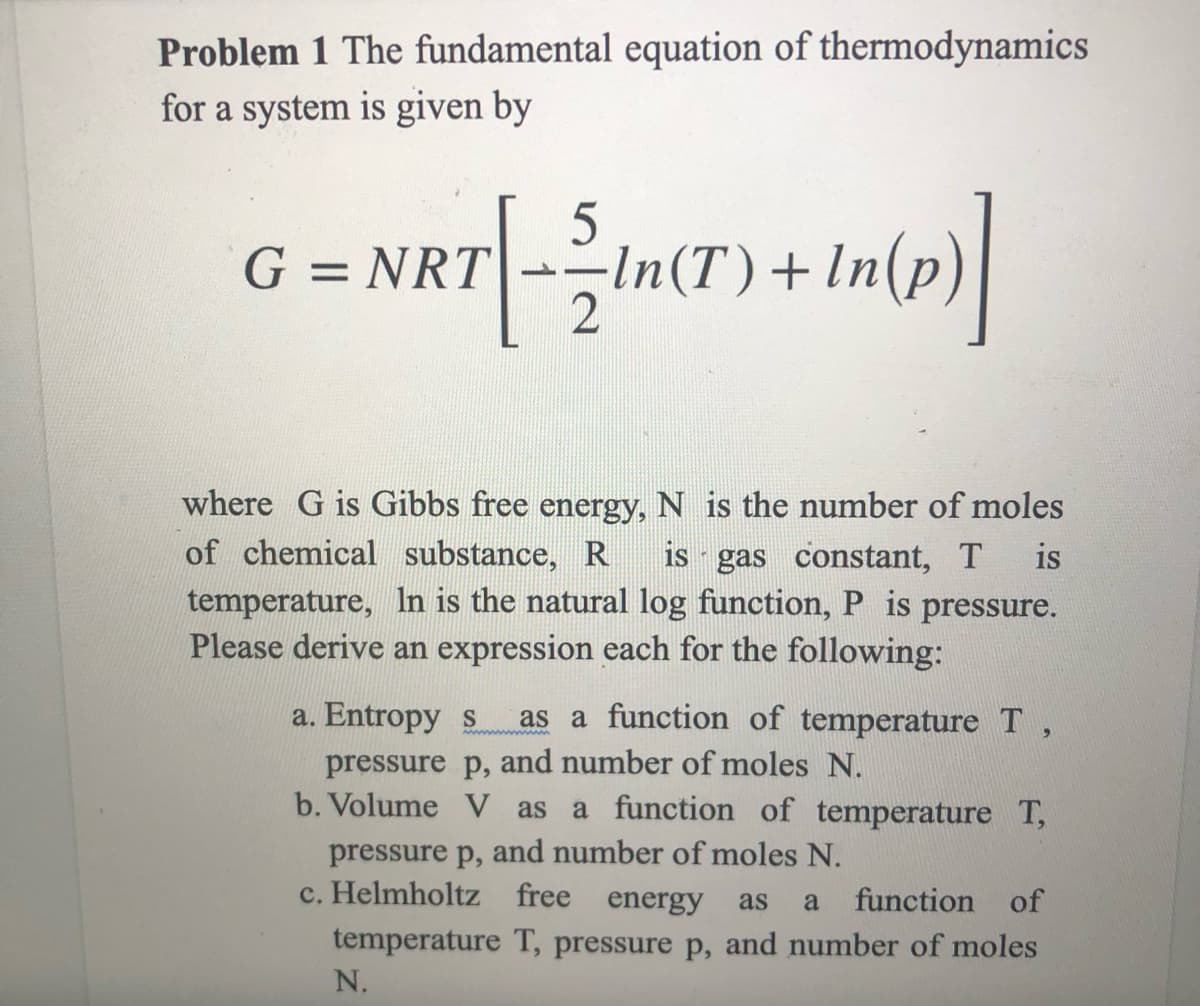
Problem 1 The fundamental equation of thermodynamics for a system is given by
Q: A group of students wish to determine, experimentally, the bulk density of an unknown metal. To dete...
A: Mass is a property of an object that determines how heavy or light it is. The volume of an object is...
Q: Questions 1-3 refer to the figure below. 0.01 m 0.015 m 91 92 93 1. In the Figure q1 = force on q1 d...
A: Answer 1 option (b) Answer 2 option (c) Answer 3 option (d) Detailed solution is attached below Tha...
Q: 1- calculate the matrix (mlp2|n > and (m|x21n> then find the matrix of kinetic and potential energy ...
A:
Q: Given the vectors Ā = 2 î + 3 j and B = 3 î - 7 j, find: %3D (a) The sum of the two vectors C = A + ...
A:
Q: DO NOT COPY FROM OTHER WEBSITES The height of a wave is 2m and its period is 2 seconds. Draw the wav...
A:
Q: Calcu late : a- How many node in laser output
A: We have to calculate the number of longitudinal modes in the laser cavity first. The condition for s...
Q: 2. Determine the latent heat of 1 English ton of ice.
A:
Q: qual charges, q, are situated at the corners of a reg e on each numeral of a clock face). What is th...
A: d) Given: 13 sided pentagon with charge q on 12 corners.
Q: Calculabe the percentage monochromatic intensity la when the light passes through the lager adecreas...
A: When light passes through some material, it interacts with the atoms present inside the material. So...
Q: An object undergoing simple harmonic motion takes 0.31 s to travel from one point of zero velocity t...
A: Concept used: When displacement is maximum, velocity becomes zero. Here potential energy is maximum ...
Q: Problem 2.1 (a) Twelve equal charges, q, are situated at the corners of a regular 12-sided polygon (...
A: Answer (a)+(c) For N sided polygon net force at centre will be zero irrespective of number of sides...
Q: Electrostatic force The magnitude of the electrostatic force between two point charges Q and q of th...
A:
Q: Organ pipe A, with both ends open, has a fundamental frequency of 320 Hz. The third harmonic of orga...
A: To solve the problem, we first write formula for harmonics of a pipe with both ends open, and for pi...
Q: Q10 The planet Saturn has a mass of 5.70 x 1026 kg and a diameter of 139820 km. A satellite in orbit...
A:
Q: A tiger leaps horizontally from a 15-m high cliff to reach an 8-m high rock, 15m away. (a) With a ta...
A:
Q: A particle with a mass of 3.9 x 10 20 kg is oscillating with simple harmonic motion with a period of...
A:
Q: A record player is spinning at 33.3 rpm. (a) How far does it turn in 2-sec? (b) When the motor is sh...
A: The angular speed ω is given by ω=∆θ∆t ∆θ is the angle rotated ∆t is the time interval 1 rpm = 2π60 ...
Q: AB and a B* particle meet and interact within a tissue. Discuss what ould be the likely outcome of t...
A: A beta particle is a high-energy, fast-moving positron or electron created by the radioactive breakd...
Q: 1- calculate the matrix (mlp2|n > and (m|x2|n > then find the matrix of kinetic and potential energy...
A: In both classical and quantum physics, the study of the simple harmonic oscillator is crucial. The r...
Q: 1. Flint vs. Crown Glasses: The difference between Flint and Crown glasses is how much they disperse...
A: Solution: 1) The amount of dispersion by the lens is measured by the parameter called Abbe's number ...
Q: What is the radius of a 235.00m length of gold wire whose resistance is 0.32 KN?
A: Given that,Length, l=235.0 mResistance, R=0.32KΩ=320ΩWe know that,Resistance, R=ρLAresistivity of go...
Q: A fluid enters an apparatus at 480 ft/s, initially the pressure of the fluid is 120 psia, the specif...
A:
Q: If the free end of the pulley rope is pulled down with an acceleration of 4 ft/s2, what will the acc...
A:
Q: 33. Assume that i = 10e-0.1 A (t in seconds). Calculate the flux d(t), at time t, of B through the i...
A: Given: i(t)=10e-0.1t Triangle base is 12 cm and height is 6 cm
Q: *3-44. A scale is constructed using the 10-kg mass, the 2-kg pan P, and the pulley and cord arrangem...
A:
Q: Guide Questions: 1. What happened when you lift the tape up? Did the marble continue to travel in th...
A: Concept used: Centripetal force is a force which makes an object move in circular motion. Accelerati...
Q: A narrow beam containing 1020 photons at 6 MeV impinges perpendicularly on a 12mm thick layer of Pb....
A:
Q: In the figure the ideal batteries have emfs E; = 4.89 V and E, = 10.2 V, the resistances are each 2....
A:
Q: A bag of wheat weighs 50 kg. To what height should it be raised so that its potential energy may be ...
A: Given that,Potential energy, P.E=9800 JouleMass, m=100 kgGravity, g=9.8 m/s2Potential energy, PE=mgh...
Q: iii) Consider a 2D square potential energy well with sides L (length) containing six electrons. The ...
A: The electrons moves in this two dimensional box, the energy of these electrons can be calculated usi...
Q: As a ladder leans against a wall it exerts a force on both the wall and ground. What are the two rea...
A: The forces acting on the ladder are 1. The gravity that acts downward. 2. The wall exerts a contac...
Q: 1- calculate the matrix (mlp2|n > and (m|x2|n > then find the matrix of kinetic and potential energy...
A:
Q: 3. For the optical system given below, an oblique incident beam through a parallel plate with a refr...
A: This is a very simple question on ray optics involving law of refraction. we will use Snell's law ex...
Q: c) In the Schwarzschild geometry, consider the following function: 5t2 – 2,2 f(r) = (2M)? where t an...
A: Concept used: Gradient for given Schwarzschild co-ordinates is given by: ∇a=∂∂tta+∂∂rra
Q: Title Acceleration deviation (8x)? Trial # (i) (m/s?) (ба, —а, -а) 1 -0.836 -0.806 -0.821 4 -0.891 -...
A: We need to find the mean of the data given above. The mean of a variable x is given by X¯=X1+X2+X3+....
Q: In the diagram shown below, the lower block is acted on by a force, F, which has a magnitude of 90.5...
A:
Q: 2 - Transform the vector A = â,+âo from cylindrical to C %3D
A: A→=a^ρ+a^ϕ is the vector in the cylindrical coordinate systemx=ρ cos ϕ (1)y=ρ sin ϕ (2)z=zsqu...
Q: How the slit in Polarizing filters passes electromagnetic waves thathave an electric field parallel ...
A: A polarizing filter contains several long chains of molecules along the axis perpendicular to its po...
Q: 1.8. Find the horizontal displacement due to the rotation of the Earth of a body dropped from a fixe...
A: Given: h=5 km
Q: Determine the maximum weight of the bucket that the wire system can support so that no single wire d...
A: Concept used: Vertical forces should balance to support weight of bucket.
Q: An edge-on spectroscopic binary is monitored throughout its orbit. The spectroscopy indicates the or...
A: In binary stars, both stars exerts gravitational force on each other, thus they rotate around a comm...
Q: Apply Kirchhoff’s laws to the resistive circuit shown in Figure P8.11 to generate two sets of simult...
A: Given Apply Kirchhoff’s laws to the resistive circuit shown in Figure P8.11 to generate two sets of ...
Q: Consider the case for the boson particle system. From 1 assembly containing thousands of groups, the...
A:
Q: 1- calculate the matrix (mlp2|n > and (m|x2|n > then find the matrix of kinetic and potential
A:
Q: *2-8. Resolve the force F, into components acting along the u and v axes and determine the magnitude...
A: Vectors are quantities that have a magnitude and a direction. Thus, vectors can be broken down into ...
Q: Biomedical Application. The parts of the body that are most sensitive to electric currents are the b...
A: Electrical Insulator is a material which doesn't readily carry electric current, it offers high resi...
Q: For the capacitor network shown in (Figure 1), the potential difference across ab is 63 V. Part E Fi...
A:
Q: A football player has an initial speed of 8 m/s and is stopped by a defensive lineman in a time inte...
A: Given that,The initial speed : u = 8 (m/sec)The final speed : v = 0 (m/sec)Time : 0.2 (sec)Here we n...
Q: The weight of the rock in the air is 4.5 N. When it is completely submerged in water, its weight is ...
A: Given Weight in the air = 4.5 N Weight in the water = 2 N
Q: The Keck Telescope (on Mauna Kea, Hawaii) has an aperture D = 10.0 m. Its Cassegrain focus has f/15....
A:
Trending now
This is a popular solution!
Step by step
Solved in 3 steps
