Problem 2: The enthalpy of a system is given by the equation H = U + PV where U is the internal energy, P = pressure, and V = volume. In addition, the internal energy, U = Q + w where Q is the heat and W is the work. Suppose we want to find the rate of change in the enthalpy at constant pressure of 1.25 atm, what is the value when heat is absorbed by the system at a rate of 45 J/s and work is done by the system at a rate of 100 J/s when the change of volume is rated at 35 x 105 m/s? 1. What is the change in heat with respect to time? 2. What is the change in internal energy of the system with respect to time? 3. What is the change in enthalpy of the system with respect to time?
Problem 2: The enthalpy of a system is given by the equation H = U + PV where U is the internal energy, P = pressure, and V = volume. In addition, the internal energy, U = Q + w where Q is the heat and W is the work. Suppose we want to find the rate of change in the enthalpy at constant pressure of 1.25 atm, what is the value when heat is absorbed by the system at a rate of 45 J/s and work is done by the system at a rate of 100 J/s when the change of volume is rated at 35 x 105 m/s? 1. What is the change in heat with respect to time? 2. What is the change in internal energy of the system with respect to time? 3. What is the change in enthalpy of the system with respect to time?
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter8: Natural Convection
Section: Chapter Questions
Problem 8.29P
Related questions
Question

Transcribed Image Text:Problem 2:
The enthalpy of a system is given by the equation H = U + PV where U is the internal energy, P = pressure, and V = volume.
In addition, the internal energy, U = Q + W where Q is the heat and W is the work. Suppose we want to find the rate of
change in the enthalpy at constant pressure of 1.25 atm, what is the value when heat is absorbed by the system at a rate
of 45 J/s and work is done by the system at a rate of 100 J/s when the change of volume is rated at 35 x 105 m/s?
1. What is the change in heat with respect to time?
2. What is the change in internal energy of the system with respect to time?
3. What is the change in enthalpy of the system with respect to time?
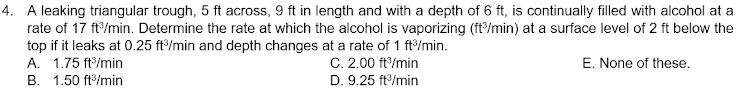
Transcribed Image Text:4. A leaking triangular trough, 5 ft across, 9 ft in length and with a depth of 6 ft, is continually filled with alcohol at a
rate of 17 ft/min. Determine the rate at which the alcohol is vaporizing (ft/min) at a surface level of 2 ft below the
top if it leaks at 0.25 ft/min and depth changes at a rate of 1 ft/min.
A. 1.75 ft/min
C. 2.00 ft/min
D. 9.25 ft/min
E. None of these.
B. 1.50 ft/min
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning