Problem 3: Consider a stationary horizontal slab of air of height dz. Since the slab is at rest, the pressure p holding it up from below must be balanced by the pressure from above PLUS the weight of the slab. a) Derive a formula for dP/dz the variation of pressure with altitude, in terms of the mass density p (greek letter rho) of the air. b) Using the ideal gas law, rewrite the air density in terms of the average mass m of the air molecules and other variables. Combine your result with part (a) to derive a differential equation relating dP/dz to P. Show then that pressure obeys the differential equation: dP тg dz kT c) Assume constant temperature T and solve the differential equation in part (b) for pressure P as a function of height z. Assume that P(z=0) is given. Show that the pressure obeys: P = P(0)e-mgz /kT Show also that the density obeys a similar equation.
Problem 3: Consider a stationary horizontal slab of air of height dz. Since the slab is at rest, the pressure p holding it up from below must be balanced by the pressure from above PLUS the weight of the slab. a) Derive a formula for dP/dz the variation of pressure with altitude, in terms of the mass density p (greek letter rho) of the air. b) Using the ideal gas law, rewrite the air density in terms of the average mass m of the air molecules and other variables. Combine your result with part (a) to derive a differential equation relating dP/dz to P. Show then that pressure obeys the differential equation: dP тg dz kT c) Assume constant temperature T and solve the differential equation in part (b) for pressure P as a function of height z. Assume that P(z=0) is given. Show that the pressure obeys: P = P(0)e-mgz /kT Show also that the density obeys a similar equation.
Related questions
Question
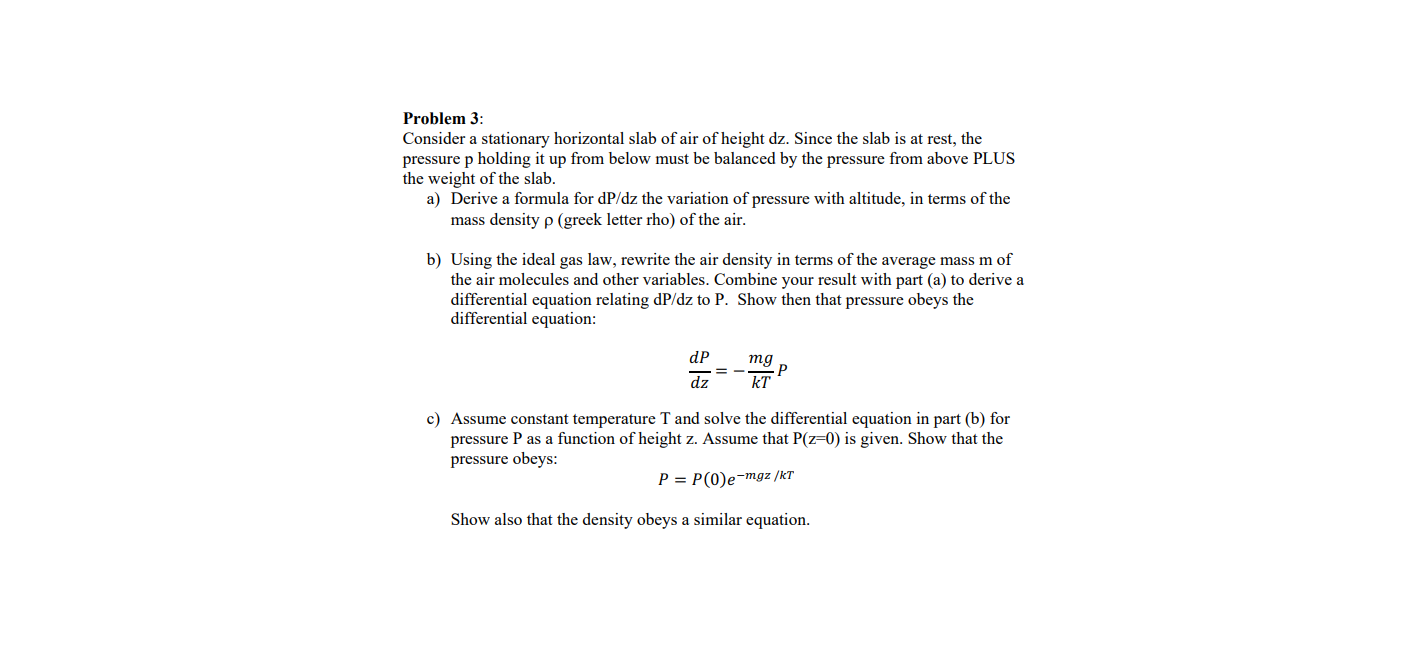
Transcribed Image Text:Problem 3:
Consider a stationary horizontal slab of air of height dz. Since the slab is at rest, the
pressure p holding it up from below must be balanced by the pressure from above PLUS
the weight of the slab.
a) Derive a formula for dP/dz the variation of pressure with altitude, in terms of the
mass density p (greek letter rho) of the air.
b) Using the ideal gas law, rewrite the air density in terms of the average mass m of
the air molecules and other variables. Combine your result with part (a) to derive a
differential equation relating dP/dz to P. Show then that pressure obeys the
differential equation:
dP
тg
dz
kT
c) Assume constant temperature T and solve the differential equation in part (b) for
pressure P as a function of height z. Assume that P(z=0) is given. Show that the
pressure obeys:
P = P(0)e-mgz /kT
Show also that the density obeys a similar equation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images
