Problem 5.8: A dry air parcel undergoes a complete Carnot cycle consisting of the steps indicated in (a)-(d). For each individual step, calculate the mechanical work w (per unit mass) done by the air par- cel and the heat q added to the parcel. = a) Adiabatic compression from p₁ temperature T2 of 25°C; Answer: w = -1.8 × 104 J kg-¹ 600 hPa and T₁ b) isothermal expansion to a pressure p3 of 700 hPa; Answer: q = 1.3 x 104 J kg-1 c) adiabatic expansion to a temperature T4 of 0°C; = 0°C to a
Problem 5.8: A dry air parcel undergoes a complete Carnot cycle consisting of the steps indicated in (a)-(d). For each individual step, calculate the mechanical work w (per unit mass) done by the air par- cel and the heat q added to the parcel. = a) Adiabatic compression from p₁ temperature T2 of 25°C; Answer: w = -1.8 × 104 J kg-¹ 600 hPa and T₁ b) isothermal expansion to a pressure p3 of 700 hPa; Answer: q = 1.3 x 104 J kg-1 c) adiabatic expansion to a temperature T4 of 0°C; = 0°C to a
Applications and Investigations in Earth Science (9th Edition)
9th Edition
ISBN:9780134746241
Author:Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Chapter1: The Study Of Minerals
Section: Chapter Questions
Problem 1LR
Related questions
Question
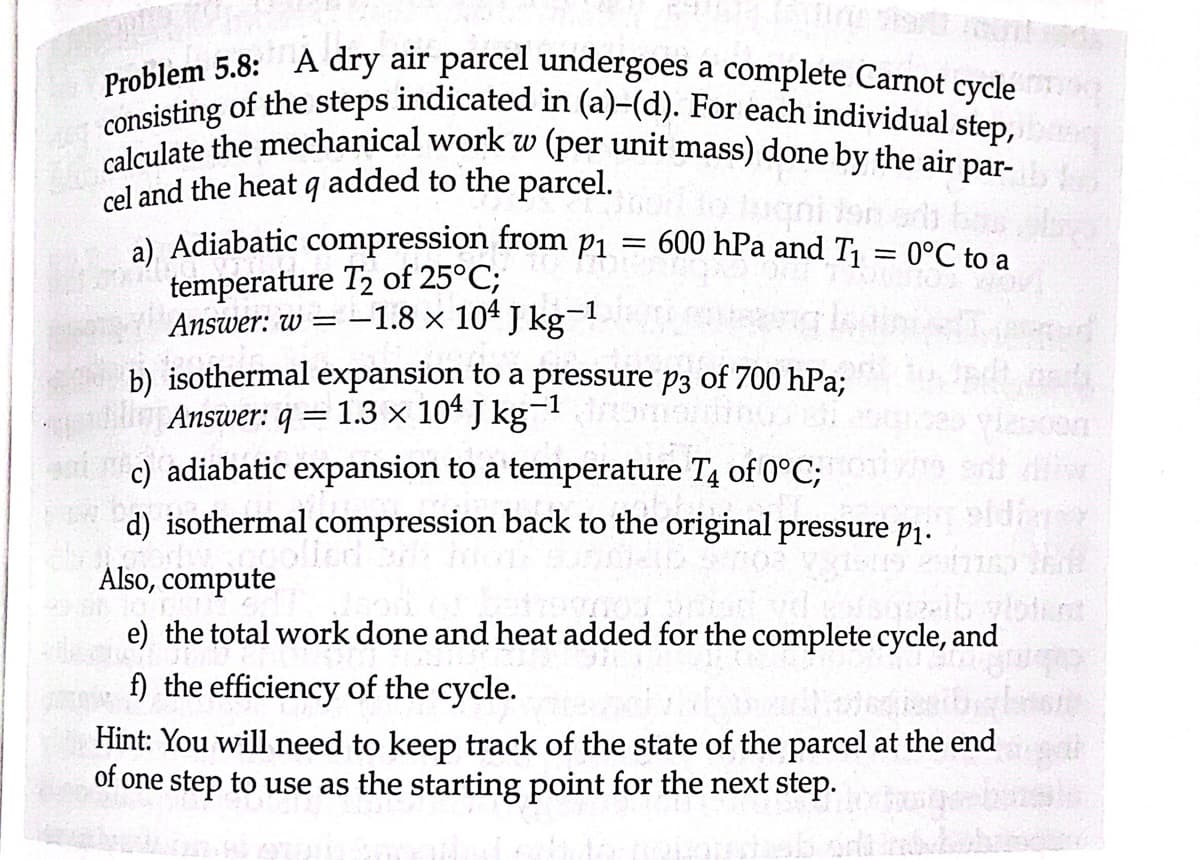
Transcribed Image Text:Problem 5.8: A dry air parcel undergoes a complete Carnot cycle
consisting of the steps indicated in (a)-(d). For each individual step,
calculate the mechanical work w (per unit mass) done by the air par-
cel and the heat q added to the parcel.
alte
a) Adiabatic compression from p₁ = 600 hPa and T₁ = 0°C to a
temperature T2 of 25°C;
.-1
Answer: w = -1.8 × 10 J kg¯
b) isothermal expansion to a pressure p3 of 700 hPa;
Answer: q = 1.3 × 104 J kg-1 droms
c) adiabatic expansion to a temperature T4 of 0°C;
htt
d) isothermal compression back to the original pressure p₁.
podwangolled
Also, compute
e) the total work done and heat added for the complete cycle, and
f) the efficiency of the cycle.
Hint: You will need to keep track of the state of the parcel at the end
of one step to use as the starting point for the next step.
vidianer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Applications and Investigations in Earth Science …
Earth Science
ISBN:
9780134746241
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Exercises for Weather & Climate (9th Edition)
Earth Science
ISBN:
9780134041360
Author:
Greg Carbone
Publisher:
PEARSON

Environmental Science
Earth Science
ISBN:
9781260153125
Author:
William P Cunningham Prof., Mary Ann Cunningham Professor
Publisher:
McGraw-Hill Education

Applications and Investigations in Earth Science …
Earth Science
ISBN:
9780134746241
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Exercises for Weather & Climate (9th Edition)
Earth Science
ISBN:
9780134041360
Author:
Greg Carbone
Publisher:
PEARSON

Environmental Science
Earth Science
ISBN:
9781260153125
Author:
William P Cunningham Prof., Mary Ann Cunningham Professor
Publisher:
McGraw-Hill Education

Earth Science (15th Edition)
Earth Science
ISBN:
9780134543536
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Environmental Science (MindTap Course List)
Earth Science
ISBN:
9781337569613
Author:
G. Tyler Miller, Scott Spoolman
Publisher:
Cengage Learning

Physical Geology
Earth Science
ISBN:
9781259916823
Author:
Plummer, Charles C., CARLSON, Diane H., Hammersley, Lisa
Publisher:
Mcgraw-hill Education,