Problem 6 Consider the Li+ ion. The atom is initially in the groundstate. a) Given that J = (Vee) z 1.50 × 10-17 J and RLi = RH, determine the (va) = ground state energy for Li+ in Joules. b) Determine the term symbol of the ground state of Lit. c) A particular excitation puts one of the electrons in the 4p state, resulting in a 1s'4p' electron configuration. What are the term symbols for this excited state? d) Write a valid triplet state wavefunction for the 1s'4p' configuration, using the Slater determinant notation.
Problem 6 Consider the Li+ ion. The atom is initially in the groundstate. a) Given that J = (Vee) z 1.50 × 10-17 J and RLi = RH, determine the (va) = ground state energy for Li+ in Joules. b) Determine the term symbol of the ground state of Lit. c) A particular excitation puts one of the electrons in the 4p state, resulting in a 1s'4p' electron configuration. What are the term symbols for this excited state? d) Write a valid triplet state wavefunction for the 1s'4p' configuration, using the Slater determinant notation.
Related questions
Question
Please answer a-d and show work
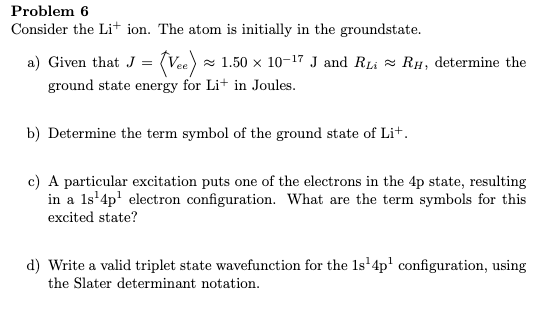
Transcribed Image Text:Problem 6
Consider the Li+ ion. The atom is initially in the groundstate.
a) Given that J = (Vee) z 1.50 × 10-17 J and RLi = RH, determine the
(va) =
ground state energy for Li+ in Joules.
b) Determine the term symbol of the ground state of Lit.
c) A particular excitation puts one of the electrons in the 4p state, resulting
in a 1s'4p' electron configuration. What are the term symbols for this
excited state?
d) Write a valid triplet state wavefunction for the 1s'4p' configuration, using
the Slater determinant notation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 7 images
