Propanone and 2-propen-1-ol are constitutional isomers. Show how to distinguish between them by IR spectroscopy. CH-C-CH, CH,— CH—CН, — ОН Propanone (Acetone) 2-Propen-1-ol (Allyl alcohol)
Propanone and 2-propen-1-ol are constitutional isomers. Show how to distinguish between them by IR spectroscopy. CH-C-CH, CH,— CH—CН, — ОН Propanone (Acetone) 2-Propen-1-ol (Allyl alcohol)
Chapter12: Structure Determination: Mass Spectrometry And Infrared Spectroscopy
Section12.SE: Something Extra
Problem 28AP: Would you expect two enantiomers such as (R)-2-bromobutane and (S)-2-bromobutane to have identical...
Related questions
Question
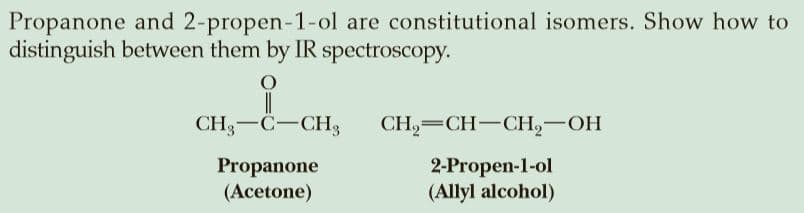
Transcribed Image Text:Propanone and 2-propen-1-ol are constitutional isomers. Show how to
distinguish between them by IR spectroscopy.
CH-C-CH,
CH,— CH—CН, — ОН
Propanone
(Acetone)
2-Propen-1-ol
(Allyl alcohol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole