Properties of pure substances Remember that since they are pure supstances, you must iook ior the properties in the Thermodynamic tables that correspond to the substance you are analyzing, so you must always have your tables when solving the exercises. a) A closed system to be analyzed contains water initially at 120 °C, 198.67 kPa as a saturated liquid. If water changes state in a thermodynamic process to saturated steam, determine its final internal energy. b) In a thermodynamic system to be analyzed, there is refrigerant 134a initially at 10 °C, 320 kPa. If a thermodynamic process is developed where the final state of the refrigerant is - 10°C and 200.74 kPa with 60% steam quality, determine its final enthalpy. c) In a thermodynamic system to be analyzed, there is water initially at 350 °C, 2000 kPa. If the water changes state in an isobaric process up to 225°C, determine its final entropy. d) Find the enthalpy change of water during the phase change from saturated liquid to saturated vapor at 175 kPa and 486.82 kJ/kg internal energy. It is: e) Find the enthalpy change of water during the phase change from saturated liquid to saturated vapor at 275 kPa and 130.58 °C. It is:
Properties of pure substances Remember that since they are pure supstances, you must iook ior the properties in the Thermodynamic tables that correspond to the substance you are analyzing, so you must always have your tables when solving the exercises. a) A closed system to be analyzed contains water initially at 120 °C, 198.67 kPa as a saturated liquid. If water changes state in a thermodynamic process to saturated steam, determine its final internal energy. b) In a thermodynamic system to be analyzed, there is refrigerant 134a initially at 10 °C, 320 kPa. If a thermodynamic process is developed where the final state of the refrigerant is - 10°C and 200.74 kPa with 60% steam quality, determine its final enthalpy. c) In a thermodynamic system to be analyzed, there is water initially at 350 °C, 2000 kPa. If the water changes state in an isobaric process up to 225°C, determine its final entropy. d) Find the enthalpy change of water during the phase change from saturated liquid to saturated vapor at 175 kPa and 486.82 kJ/kg internal energy. It is: e) Find the enthalpy change of water during the phase change from saturated liquid to saturated vapor at 275 kPa and 130.58 °C. It is:
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 29RQ: If 3000 ft3 of air is crossing an evaporator coil and iscooled from 75F to 55F, what would be the...
Related questions
Question
please step by step, and the procedure clear, clear handwriting, I thank you very much for your work
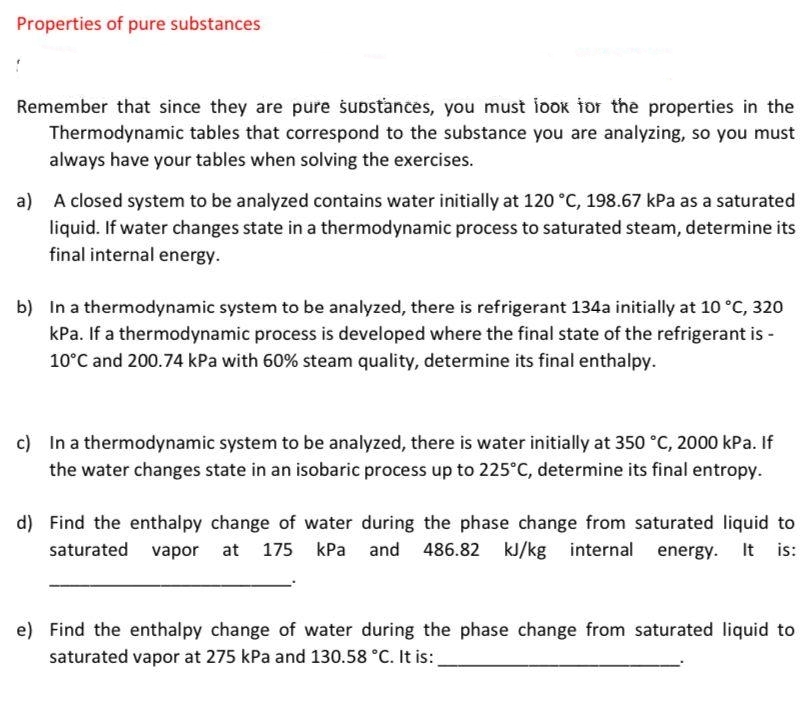
Transcribed Image Text:Properties of pure substances
Remember that since they are pure supstances, you must iook ior the properties in the
Thermodynamic tables that correspond to the substance you are analyzing, so you must
always have your tables when solving the exercises.
a) A closed system to be analyzed contains water initially at 120 °C, 198.67 kPa as a saturated
liquid. If water changes state in a thermodynamic process to saturated steam, determine its
final internal energy.
b) In a thermodynamic system to be analyzed, there is refrigerant 134a initially at 10 °C, 320o
kPa. If a thermodynamic process is developed where the final state of the refrigerant is -
10°C and 200.74 kPa with 60% steam quality, determine its final enthalpy.
c) In a thermodynamic system to be analyzed, there is water initially at 350 °C, 2000 kPa. If
the water changes state in an isobaric process up to 225°C, determine its final entropy.
d) Find the enthalpy change of water during the phase change from saturated liquid to
saturated
vapor at 175 kPa and 486.82 kJ/kg internal energy. It is:
e) Find the enthalpy change of water during the phase change from saturated liquid to
saturated vapor at 275 kPa and 130.58 °C. It is:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning