Provide an explanation for the way the following formula Ca5(PO4)3OH is written and state the name of all the ions involved. (Explain what the subscripted numbers indicate, why is PO4 in brackets whereas OH isn’t?)
Provide an explanation for the way the following formula Ca5(PO4)3OH is written and state the name of all the ions involved. (Explain what the subscripted numbers indicate, why is PO4 in brackets whereas OH isn’t?)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 52E: Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 6.00...
Related questions
Question
Provide an explanation for the way the following formula Ca5(PO4)3OH is written and state the name of all the ions involved. (Explain what the subscripted numbers indicate, why is PO4 in brackets whereas OH isn’t?)
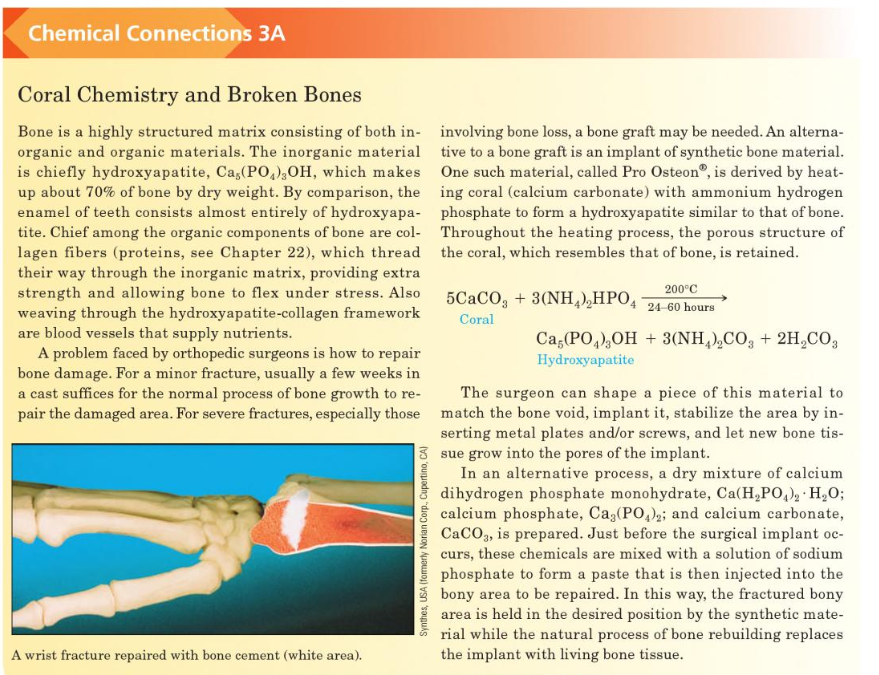
Transcribed Image Text:Chemical Connections 3A
Coral Chemistry and Broken Bones
Bone is a highly structured matrix consisting of both in- involving bone loss, a bone graft may be needed. An alterna-
organic and organic materials. The inorganic material tive to a bone graft is an implant of synthetic bone material.
is chiefly hydroxyapatite, Ca,(PO,),OH, which makes One such material, called Pro Osteon®, is derived by heat-
up about 70% of bone by dry weight. By comparison, the ing coral (calcium carbonate) with ammonium hydrogen
enamel of teeth consists almost entirely of hydroxyapa- phosphate to form a hydroxyapatite similar to that of bone.
tite. Chief among the organic components of bone are col- Throughout the heating process, the porous structure of
lagen fibers (proteins, see Chapter 22), which thread the coral, which resembles that of bone, is retained.
their way through the inorganic matrix, providing extra
strength and allowing bone to flex under stress. Also
weaving through the hydroxyapatite-collagen framework
are blood vessels that supply nutrients.
A problem faced by orthopedic surgeons is how to repair
bone damage. For a minor fracture, usually a few weeks in
a cast suffices for the normal process of bone growth to re-
200°C
5CACO, + 3(NH,),HPO, 24-60 hours
Coral
Ca,(PO,),OH + 3(NH,),CO, + 2H,CO3
Hydroxyapatite
The surgeon can shape a piece of this material to
pair the damaged area. For severe fractures, especially those match the bone void, implant it, stabilize the area by in-
serting metal plates and/or screws, and let new bone tis-
3 sue grow into the pores of the implant.
In an alternative process, a dry mixture of calcium
dihydrogen phosphate monohydrate, Ca(H,PO,), H,0O;
calcium phosphate, Ca3(PO,); and calcium carbonate,
CaCO3, is prepared. Just before the surgical implant oc-
curs, these chemicals are mixed with a solution of sodium
phosphate to form a paste that is then injected into the
bony area to be repaired. In this way, the fractured bony
area is held in the desired position by the synthetic mate-
rial while the natural process of bone rebuilding replaces
A wrist fracture repaired with bone cement (white area).
the implant with living bone tissue.
Synthes, USA (fomerly Norian Corp., Cupertino, CA)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax