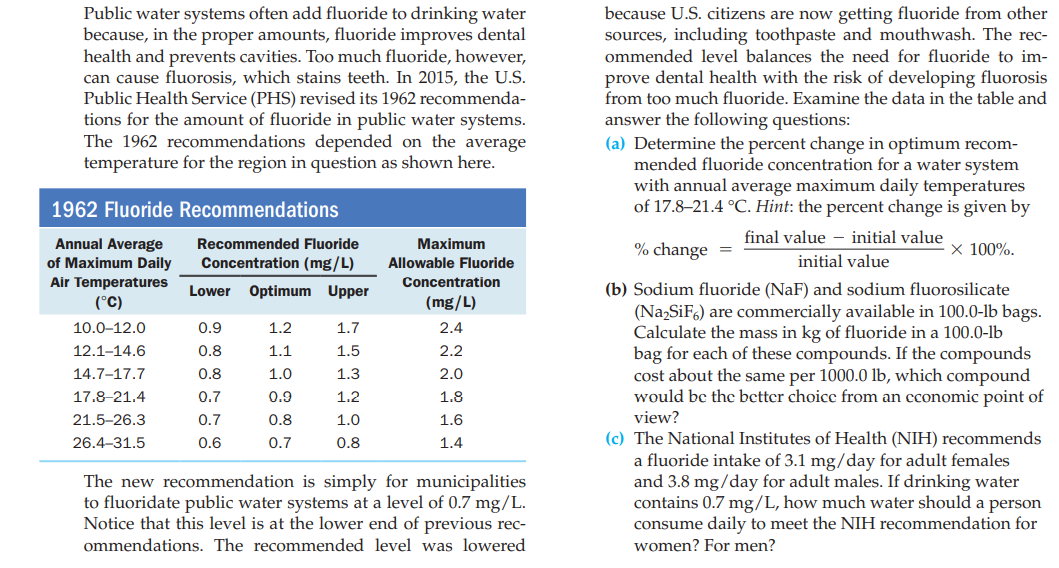
Public water systems often add fluoride to drinking water because, in the proper amounts, fluoride improves dental health and prevents cavities. Too much fluoride, however, can cause fluorosis, which stains teeth. In 2015, the U.S. Public Health Service (PHS) revised its 1962 recommenda- tions for the amount of fluoride in public water systems. The 1962 recommendations depended on the average temperature for the region in question as shown here. because U.S. citizens are now getting fluoride from other sources, including toothpaste and mouthwash. The rec- ommended level balances the need for fluoride to im- prove dental health with the risk of developing fluorosis from too much fluoride. Examine the data in the table and answer the following questions: (a) Determine the percent change in optimum recom- mended fluoride concentration for a water system with annual average maximum daily temperatures of 17.8–21.4 °C. Hint: the percent change is given by 1962 Fluoride Recommendations final value – initial value Annual Average of Maximum Daily Air Temperatures (°C) Recommended Fluoride Maximum % change = x 100%. Concentration (mg/L) Allowable Fluoride initial value Concentration (b) Sodium fluoride (NaF) and sodium fluorosilicate (Na,SiF6) are commercially available in 100.0-lb bags. Calculate the mass in kg of fluoride in a 100.0-lb bag for each of these compounds. If the compounds cost about the same per 1000.0 lb, which compound would bc the better choice from an cconomic point of Lower Optimum Upper (mg/L) 10.0–12.0 0.9 1.2 1.7 2.4 12.1–14.6 0.8 1.1 1.5 2.2 14.7-17.7 0.8 1.0 1.3 2.0 17.8-21.4 0.7 0.9 1.2 1.8 view? (c) The National Institutes of Health (NIH) recommends a fluoride intake of 3.1 mg/day for adult females and 3.8 mg/day for adult males. If drinking water contains 0.7 mg/L, how much water should a person consume daily to meet the NIH recommendation for women? For men? 21.5-26.3 0.7 0.8 1.0 1.6 26.4-31.5 0.6 0.7 0.8 1.4 The new recommendation is simply for municipalities to fluoridate public water systems at a level of 0.7 mg/L. Notice that this level is at the lower end of previous rec- ommendations. The recommended level was lowered
Public water systems often add fluoride to drinking water because, in the proper amounts, fluoride improves dental health and prevents cavities. Too much fluoride, however, can cause fluorosis, which stains teeth. In 2015, the U.S. Public Health Service (PHS) revised its 1962 recommenda- tions for the amount of fluoride in public water systems. The 1962 recommendations depended on the average temperature for the region in question as shown here. because U.S. citizens are now getting fluoride from other sources, including toothpaste and mouthwash. The rec- ommended level balances the need for fluoride to im- prove dental health with the risk of developing fluorosis from too much fluoride. Examine the data in the table and answer the following questions: (a) Determine the percent change in optimum recom- mended fluoride concentration for a water system with annual average maximum daily temperatures of 17.8–21.4 °C. Hint: the percent change is given by 1962 Fluoride Recommendations final value – initial value Annual Average of Maximum Daily Air Temperatures (°C) Recommended Fluoride Maximum % change = x 100%. Concentration (mg/L) Allowable Fluoride initial value Concentration (b) Sodium fluoride (NaF) and sodium fluorosilicate (Na,SiF6) are commercially available in 100.0-lb bags. Calculate the mass in kg of fluoride in a 100.0-lb bag for each of these compounds. If the compounds cost about the same per 1000.0 lb, which compound would bc the better choice from an cconomic point of Lower Optimum Upper (mg/L) 10.0–12.0 0.9 1.2 1.7 2.4 12.1–14.6 0.8 1.1 1.5 2.2 14.7-17.7 0.8 1.0 1.3 2.0 17.8-21.4 0.7 0.9 1.2 1.8 view? (c) The National Institutes of Health (NIH) recommends a fluoride intake of 3.1 mg/day for adult females and 3.8 mg/day for adult males. If drinking water contains 0.7 mg/L, how much water should a person consume daily to meet the NIH recommendation for women? For men? 21.5-26.3 0.7 0.8 1.0 1.6 26.4-31.5 0.6 0.7 0.8 1.4 The new recommendation is simply for municipalities to fluoridate public water systems at a level of 0.7 mg/L. Notice that this level is at the lower end of previous rec- ommendations. The recommended level was lowered
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 49RGQ: Fluoridation of city water supplies has been practiced in the United States for several decades. It...
Related questions
Question
Transcribed Image Text:Public water systems often add fluoride to drinking water
because, in the proper amounts, fluoride improves dental
health and prevents cavities. Too much fluoride, however,
can cause fluorosis, which stains teeth. In 2015, the U.S.
Public Health Service (PHS) revised its 1962 recommenda-
tions for the amount of fluoride in public water systems.
The 1962 recommendations depended on the average
temperature for the region in question as shown here.
because U.S. citizens are now getting fluoride from other
sources, including toothpaste and mouthwash. The rec-
ommended level balances the need for fluoride to im-
prove dental health with the risk of developing fluorosis
from too much fluoride. Examine the data in the table and
answer the following questions:
(a) Determine the percent change in optimum recom-
mended fluoride concentration for a water system
with annual average maximum daily temperatures
of 17.8–21.4 °C. Hint: the percent change is given by
1962 Fluoride Recommendations
final value – initial value
Annual Average
of Maximum Daily
Air Temperatures
(°C)
Recommended Fluoride
Maximum
% change =
x 100%.
Concentration (mg/L)
Allowable Fluoride
initial value
Concentration
(b) Sodium fluoride (NaF) and sodium fluorosilicate
(Na,SiF6) are commercially available in 100.0-lb bags.
Calculate the mass in kg of fluoride in a 100.0-lb
bag for each of these compounds. If the compounds
cost about the same per 1000.0 lb, which compound
would bc the better choice from an cconomic point of
Lower Optimum Upper
(mg/L)
10.0–12.0
0.9
1.2
1.7
2.4
12.1–14.6
0.8
1.1
1.5
2.2
14.7-17.7
0.8
1.0
1.3
2.0
17.8-21.4
0.7
0.9
1.2
1.8
view?
(c) The National Institutes of Health (NIH) recommends
a fluoride intake of 3.1 mg/day for adult females
and 3.8 mg/day for adult males. If drinking water
contains 0.7 mg/L, how much water should a person
consume daily to meet the NIH recommendation for
women? For men?
21.5-26.3
0.7
0.8
1.0
1.6
26.4-31.5
0.6
0.7
0.8
1.4
The new recommendation is simply for municipalities
to fluoridate public water systems at a level of 0.7 mg/L.
Notice that this level is at the lower end of previous rec-
ommendations. The recommended level was lowered
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning