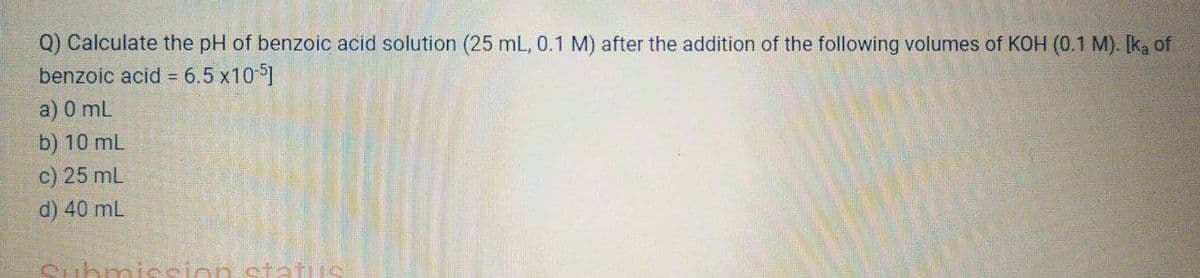
Q) Calculate the pH of benzoic acid solution (25 mL, 0.1 M) after the addition of the following volumes of KOH (0.1 M). [ka of benzoic acid = 6.5 x10 1 a) 0 mL b) 10 mL c) 25 mL d) 40 mL Submiesion status
Q: situation where inflammation is good and
A:
Q: Q:-Do you think microbes can also be used as a source of energy? If yes, how?
A: Microbes, like bacteria and fungus, are minute, single-celled creatures. Despite their association…
Q: What are some organizations doing to prevent the spread of malaria?
A: The Malarial parasite, Plasmodium vivax belonging to the Genus, Plasmodium, possess a life cycle…
Q: 5. After extracting your DNA from your cheek cells, we will add a solution called Master Mix to your…
A: PCR abbreviated as Polymerase Chain Reaction is often used in the research and medical field and is…
Q: What is meant by the term ‘breed’? What are the objectives of animal breeding?
A: The group of animals having the same ancestry characters, general appearance, and size are called…
Q: Health risk of exposure to current Bisphenol A (BPA)
A: BPA BPA or bisphenol A refers to an industrial chemical. This chemical is present in polycarbonate…
Q: Anita has been feeling extremely tired, shaky, and confused. Her doctor has diagnosed her with low…
A: Blood sugar level can be described as the concentration of blood sugar in the blood of humans or…
Q: Discuss factors that might influence the activity of DNA-binding proteins (transcription factors)…
A: *Gene expression is the process through which information from a gene is used to create a…
Q: If your family owned a dairy farm, what measures would you undertake to improve the quality and…
A: Dairy farm management, also known as dairying, refers to the process of raising animals for the…
Q: Identify the groups to which each of the following plants belongs. List the characteristics you can…
A: Begonia fimbriata . Begonia 'Fimbriata Ruffled Red' is an indefatigable bloomer producing myriads of…
Q: Please list and briefly describe the steps occuring on each side of the following:
A: We know that meiosis is a specialized form of cell division in in which number of chromosomes is…
Q: IV. Heterosis. Records from a dairy farm is presented. Evaluate the effect of crossbreeding on the…
A: The application of scientific and technical principles to the processing of the material by…
Q: 2. In 1665 Robert Hooke, an English scientist, published his book Micrographia, in which he…
A: Eukaryotes are the organism whi h comprises of true nucleus and membrane bound organelles. It…
Q: What are the differences between the life cycle of Symbion Pandora and Plasmodium (commonly known as…
A: Symbian Pandora and Plasmodium species,both can cause malaria, hence known as malarial parasites.
Q: Which is NOT a source of genetic variation in sexually reproducing organisms? Independent assortment…
A: Sexually reproducing organisms are the organisms that produce sex cells or gamates. The fusion of…
Q: Compare and contrast the advantages and disadvantages of production of genetically modified crops.
A: Genetically modified crops are those crops in which genes have been altered in order to bring a…
Q: elate the terms presented in the first column with the information in the second column; i.…
A: The adhesion of atoms, ions, or molecules from a gas, liquid, or dissolved solid to a surface is…
Q: Considering all you have read regarding nemertines, what phylum do you think is their closest…
A: During the course of evolution animals with Slender and cylindrical bodies were created and these…
Q: 9. Regarding the gray matter in the spinal cord, all of the following are correct EXCEPT which one?…
A: The gray matter is hornlike structure in the inside of spinal cord and white matter is the one that…
Q: Q:-Do you think microbes can also be used as a source of energy? If yes, how?
A: Yes, microbes can be used as a source of energy. Bacteria such as Methane bacterium is used for the…
Q: Allergic reactions to penicillins are considered a/an hypersensitivity. T-cell-mediated O…
A: Hypersensitivity is a condition in which the normally protective immune system has harmful effect on…
Q: How can you confirm yeast growing on Sabouraud dextrose agar to be Candida albicans?
A: Introduction : Candida albicans is the most well-known fungus that attacks the human mucosal layer.…
Q: Your science teacher says that matter is conserved in the process of cellular respiration. What…
A: Cellular respiration is a process in which glucose and oxygen molecules react to produce water,…
Q: 109
A: Mitosis and meiosis are the two kinds of cell division. Once we say "cell division," they usually…
Q: If acetylcholine re-uptake is blocked in the synaptic cleft, then what happens in the muscle cell?…
A: "Muscle contraction" is responsible for every movement in our bodies and may be found in every…
Q: Radiometric dating and the cranial capacity measurements are back from the paleolab for the specimen…
A: Introduction Osteology is a branch of anatomy that deals with the study of bones. The body skeleton…
Q: What is apiculture? How is it important in our lives?
A: Apiculture is the practise of keeping and rearing honey bees (Apis indica) in order to produce…
Q: What is one difference between fermentation and anaerobic respiration? Since neither requires…
A: Respiration There are three types of processes of oxidation of food substrates in different…
Q: Type of Medium Original Color Any Chemicals Added to Visualize Reaction? Negative Result Positive…
A: Phenol red Glucose original colour will be yellow. Negative results indicates that there is drop in…
Q: Function-based definitions of aging ? are perfect and have no limitations identify processes…
A: Aging is the sequential or progressive change in an organism that leads to an increased risk of…
Q: Compare and contrast the advantages and disadvantages of production of genetically modified crops.
A: Genetically modified crops are those whose genetic material has been altered in such a way that does…
Q: Week 1 (lessons 1 and 2) 1. Explain how to properly use a micropipette (tips, drawing samples,…
A: Micropipette is an important laboratory tool used for transferring liquid in a very small amount of…
Q: Examine the pTrcHis expression vector from Invitrogen. What is lacI q ? Why is it found on the…
A: In a molecular cloning method, a vector can be referred to as any vehicle, most often a plasmid,…
Q: Calculate the allelic frequencies observed in the class for marker PV92. Is the class 'population'…
A: Key points: Microevolution is a change in the frequency of gene variants, alleles, in a…
Q: How Can Fragments of DNA Be Separated From One Another? Agarose gel electrophoresis is a procedure…
A: Note- as we are allowed to only 3 subparts of a question, i can provide answers for first three…
Q: If both parents are heterozygous Aa. If the first child is albino what is the probability that the…
A: Albinism occurs when both the alleles are homozygous recessive. Genotype of parent 1 = Aa Genotype…
Q: The first column of the table below shows the beginning of a gene and five different mutations of…
A: Codon is a sequence of three nucleotides that codes for specific amino acid. Codons encode amino…
Q: Discuss, with examples, the similarities and differences between mechanisms of resistance to…
A: The term xenobiotic refers to chemical substances that are alien to animal life. Environmental…
Q: THE CODONS THAT CODE FOR THE AMIMO ACID SER (SERINE) ARE UCU, UCC,_____ AND _____
A: Codon: A three-nucleotide sequence in DNA or RNA that encodes a protein amino acid or indicates the…
Q: What is the color of kernels with CICRRPPYY genotype? Yellow White Purple Red
A: * The genotype of an organism can be defined as the complete set of genetic material used to refer…
Q: This is the full question... "Both the cytokinin receptor encoded by CRE1 and the ethylene receptor…
A:
Q: How is Indian ink used to demonstrate Cryptococcus neoformas? What reaction takes place between the…
A:
Q: 4. Sickle cell anemia occurs due to a point mutation in a gene for hemoglobin protein. This mutation…
A: Under normal conditions, the amino acid formation would be as below - DNA gene - CTC mRNA - GAG…
Q: Question 2 If a person suffers a chest wound that allows air from the outside to leak into the…
A: ANSWER;- Lungs are the respiratory organs present in the ribcage of the thoracic cavity lined by…
Q: Two human parents with dimples have children and they all do not have dimples. What are the…
A: Dimples are dominant traits. Dominant traits arise from those alleles (dominant) which are always…
Q: ue or False: Most fungi secondarily lack flagellated cells in their life history and this places the…
A: A plethora of organisms inhabit the whole Earth. These organisms function at different…
Q: Question 5 Eukaryotic transcription regulators can control transcription initiation away from the…
A: Transcription is the process of RNA production from the DNA template that occurs within the nucleus…
Q: Can you think and answer how a reporter enzyme can be used to monitor transformation of host cells…
A: An example of a reporter gene is the Lac Z gene, this gene encodes for fluorescent proteins in the…
Q: 1. Would you consider annelids as successful in the terrestrial environment? Why or why not? 2.…
A: Annelids are the phylum of the animal kingdom that are are found nearly everywhere on earth. They…
Q: Why don't all flowers have petals of the same color? Choose all that apply O Each pollinator has…
A: Angiosperms produce flowers with different colours. Actually the petals of the flowers have…
Step by step
Solved in 3 steps

- How many milliliters of 0.0050 N KOH are required to neutralize 25 mL of 0.0050 N H2SO4? To neutralize 25 mL of 0.0050 M H2SO4?Consider a buffer solution that contains 0.55 M NH2CH2CO2H and 0.35 M NH2CH2CO2Na. pKa(NH2CH2CO2H)=9.88. a. Calculate its pH. b. Calculate the change in pH if 0.155 g of solid NaOH is added to 250 mL of this solution. c. If the acceptable buffer range of the solution is ±0.10 pH units, calculate how many moles of H3O+ can be neutralized by 250 mL of the initial buffer.To make up a solution of phosphate buffered saline (PBS), you need 10 mM Na2HPO4 (anhydrous) (FW: 141.96 g/mol), 3 M NaCl (FW: 58.44 g/mol), and 5mM KH2PO4 (FW: 136.09 g/mol). How many grams of each will you need to make up 850 mL of PBS?
- Describe the preparation of 2.00 L of 0.100 M glycine buffer, pH 9.0, from glycine and 1.00 M NaOH. What mass of glycine is required, and what volume of 1.00 NaOH is required? The appropriate pKa of glycine is 9.6Calculate the cocentrations of acetic acid (pKa = 4.76) and sodium acetate necessary to prepare a 0.2 M buffer solution at pH 5.0.If an unknown solution of cobalt (II) chloride has an absorbance of 0.79, what is its concentration? Include proper units, please How did you determine this using the Beer’s Law plot?
- Calculate pNF concentration in each cuvette using Beer-Lambert’s Law. ε = 18,000 M-1 cm-1; b = 2.00 cm Cuvette 1 pNF 1 mL Water 2 mL Absorbance 0.546Calculate the pH of a mixture of 0.25 M acetic acid and 0.20 M sodium acetate. The pKa of acetic acid is 4.76.Calculate acetic acid when it requires 44.82mL of a 0.145M NaOH solution to titrate 34.95 mL of our unknown acetic acid

