Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter7: Extraction
Section: Chapter Questions
Problem 1Q
Related questions
Question
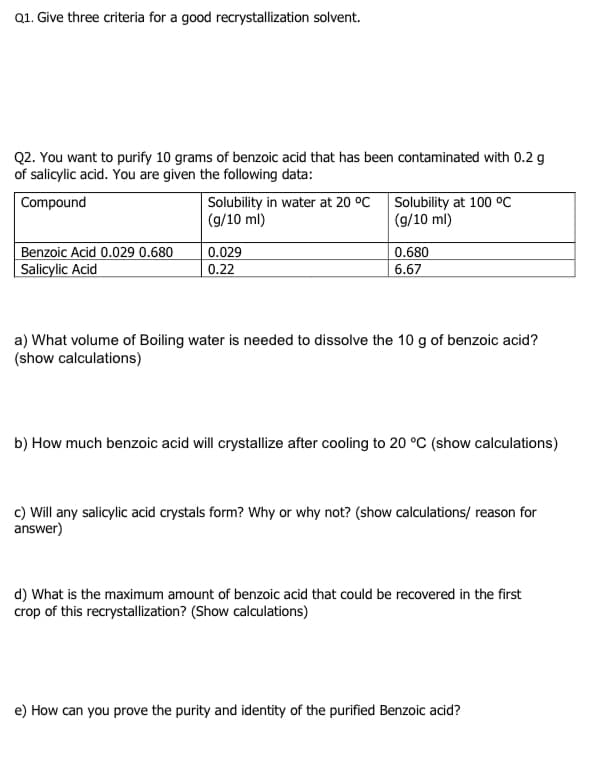
Transcribed Image Text:Q1. Give three criteria for a good recrystallization solvent.
Q2. You want to purify 10 grams of benzoic acid that has been contaminated with 0.2 g
of salicylic acid. You are given the following data:
Solubility in water at 20 °C Solubility at 100 °C
(g/10 ml)
Compound
(g/10 ml)
Benzoic Acid 0.029 0.680
Salicylic Acid
0.680
0.029
0.22
6.67
a) What volume of Boiling water is needed to dissolve the 10 g of benzoic acid?
(show calculations)
b) How much benzoic acid will crystallize after cooling to 20 °C (show calculations)
c) Will any salicylic acid crystals form? Why or why not? (show calculations/ reason for
answer)
d) What is the maximum amount of benzoic acid that could be recovered in the first
crop of this recrystallization? (Show calculations)
e) How can you prove the purity and identity of the purified Benzoic acid?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole