Q3/A 0.80868 gm sample of a commercial phosphate detergent was ignited at a red heat to destroy the organic matter the residue was then taken up in hot HCl, which converted the P to H3PO4. The phosphate was precipitated as MgNH.PO4.6H₂O by addition of Mg¹2 followed by aqueous NH3. after being filtered and washed. The precipitate was converted to Mg₂P₂0 (222.57gm mol) by ignition at 1000 Cº this residue weighed represent 61.5% of the total sample mass. Calculate the percent P(30.974gm mol),0 (16 gm/mol) and Mg (24.3 gm/mol) in the sample.
Q3/A 0.80868 gm sample of a commercial phosphate detergent was ignited at a red heat to destroy the organic matter the residue was then taken up in hot HCl, which converted the P to H3PO4. The phosphate was precipitated as MgNH.PO4.6H₂O by addition of Mg¹2 followed by aqueous NH3. after being filtered and washed. The precipitate was converted to Mg₂P₂0 (222.57gm mol) by ignition at 1000 Cº this residue weighed represent 61.5% of the total sample mass. Calculate the percent P(30.974gm mol),0 (16 gm/mol) and Mg (24.3 gm/mol) in the sample.
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.19QAP
Related questions
Question
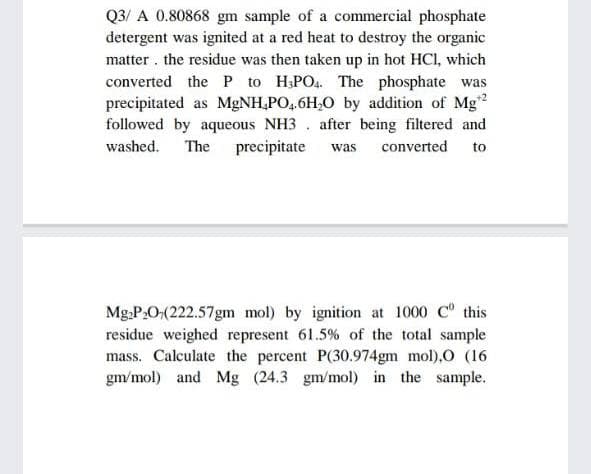
Transcribed Image Text:Q3/ A 0.80868 gm sample of a commercial phosphate
detergent was ignited at a red heat to destroy the organic
matter the residue was then taken up in hot HCl, which
converted the P to H3PO4. The phosphate was
precipitated as MgNH₂PO4.6H₂O by addition of Mg¹2
followed by aqueous NH3 after being filtered and
washed. The precipitate was converted to
Mg₂P₂O(222.57gm mol) by ignition at 1000 C this
residue weighed represent 61.5% of the total sample
mass. Calculate the percent P(30.974gm mol),O (16
gm/mol) and Mg (24.3 gm/mol) in the sample.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole