Q8. Convert 76.8 cm to m. (a) 0.0768 m (c) 0.768 m Q9. Convert 2855 mg to kg. (a) 2.855 x 10³ kg (b) 2.855 kg (c) 0.02855 kg (d) 3.503 x 104 kg Chemical Principles (b) 7.68 m (d) 7.68 x 10-2 m P:91 'e:g1 'qt
Q8. Convert 76.8 cm to m. (a) 0.0768 m (c) 0.768 m Q9. Convert 2855 mg to kg. (a) 2.855 x 10³ kg (b) 2.855 kg (c) 0.02855 kg (d) 3.503 x 104 kg Chemical Principles (b) 7.68 m (d) 7.68 x 10-2 m P:91 'e:g1 'qt
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.98QE
Related questions
Question
100%
Q9. I am unsure on how to get the final answer. I believe we have to convert the number to scientific notation but I’m not sure how they got that answer.
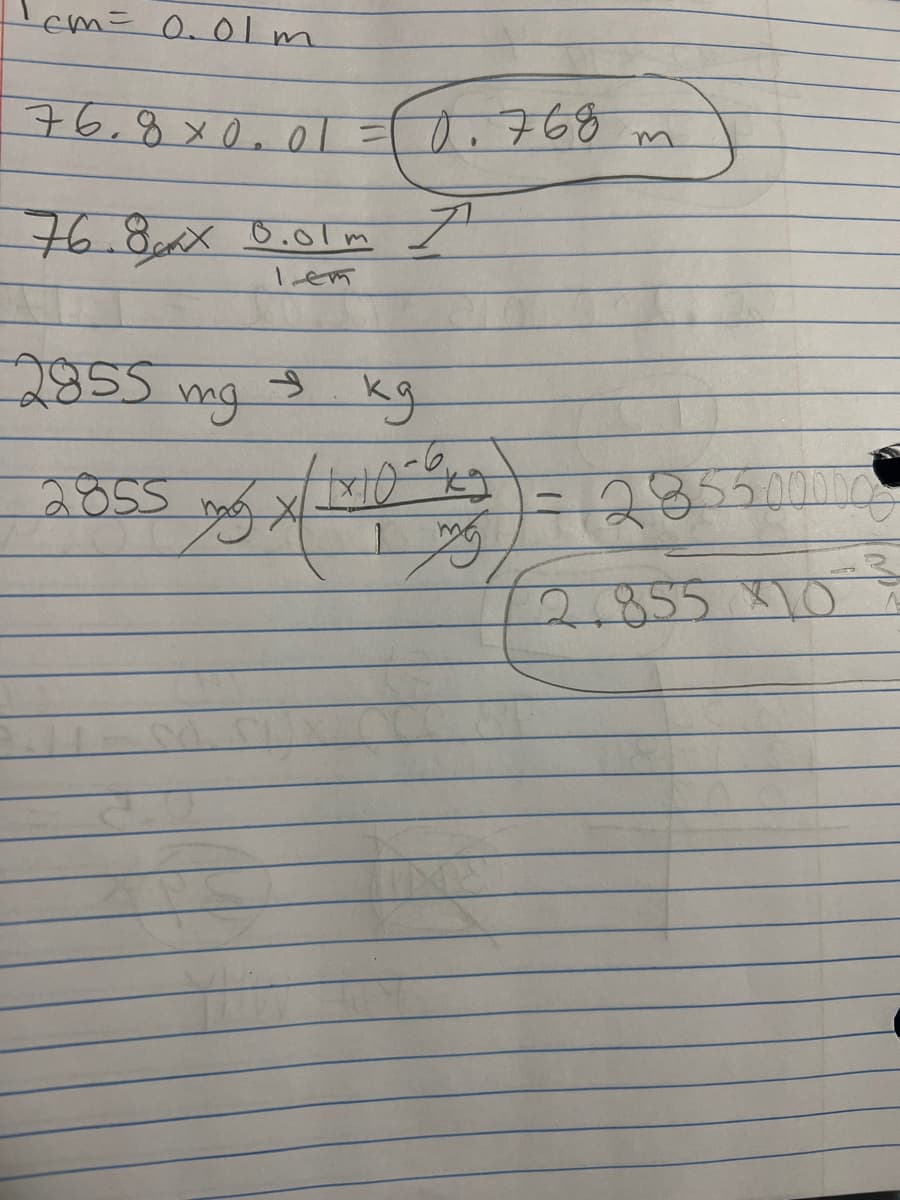
Transcribed Image Text:cm = 0.01m
76.8×0.01 = 10.768
76.8xx 0.01m I
lem
2855
S
kg
11x10-0x5²
179/
mg
2855
mg
ng x
= 285500000
2.855 10

Transcribed Image Text:r
(a) 1.17
(b) 1.18
(c) 1.2
(d) 1.176
Q6. Perform this addition to the correct number of significant
figures: 8.32 + 12.148 +0.02
(a) 20.488 (b) 20.49
(c) 20.5
(d) 21
Q7. Perform this calculation to the correct number of
significant figures: 78.222 x (12.02 - 11.52)
(a) 39
(b) 39.1
(c) 39.11
(d) 39.111
Q8. Convert 76.8 cm to m.
(a) 0.0768 m
(c) 0.768 m
Q9. Convert 2855 mg to kg.
(a) 2.855 x 10-³ kg
(b) 2.855 kg
(c) 0.02855 kg
(d) 3.503 x 104 kg
p:91 'est 'q:t
Chemical Principles
Uncertainty
Scientists report measured quantities so that the number of
digits reflects the certainty in the measurement. Write mea-
sured quantities so that every digit is certain except the last,
which is estimated.
Units
Measured quantities usually have units associated with them
The SI unit for length is the meter; for mass, the kilogram; anc
for time, the second. Prefix multipliers such as kilo- or milli
om often used in combination with these basic units. The S
d to the third powe
(b) 7.68 m
(d) 7.68 x 10-² m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning