Quantum numbers arise naturally from the mathematics used to describe the possible states of an electron in an atom. The four quantum numbers, the principal quantum number (n), the angular momentum quantum number (€) , the magnetic quantum number (me), and the spin quantum number (m,) have strict rules which govern the possible values. Identify all allowable combinations of quantum numbers for an electron. O n = 3, € = 1, mẹ = 1, mş = - O n = 5, l = 5, mẹ = 0, mş = – On = 2, € = 1, mẹ = 0, m, = -1 On = 5, € = 2, mẹ = 2, ms = - In = 3, € = -1, mẹ = 1, mş = O n = 4, € = 0, mẹ = 1, mş = -
Quantum numbers arise naturally from the mathematics used to describe the possible states of an electron in an atom. The four quantum numbers, the principal quantum number (n), the angular momentum quantum number (€) , the magnetic quantum number (me), and the spin quantum number (m,) have strict rules which govern the possible values. Identify all allowable combinations of quantum numbers for an electron. O n = 3, € = 1, mẹ = 1, mş = - O n = 5, l = 5, mẹ = 0, mş = – On = 2, € = 1, mẹ = 0, m, = -1 On = 5, € = 2, mẹ = 2, ms = - In = 3, € = -1, mẹ = 1, mş = O n = 4, € = 0, mẹ = 1, mş = -
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter2: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 5RQ: What are quantum numbers? What information do we get from the quantum numbers n, l, and ml? We...
Related questions
Question
Quantum numbers arise naturally from the mathematics used to describe the possible states of an electron in an atom. The four quantum numbers, the principal quantum number (?), the angular momentum quantum number (?), the magnetic quantum number (??), and the spin quantum number (?s) have strict rules which govern the possible values.
Identify all allowable combinations of quantum numbers for an electron.
Complete the table by pairing each set of quantum numbers with the orbital it describes. If the set of quantum numbers is not possible, label it as not allowed. Use each orbital description as many times as necessary.

Transcribed Image Text:Quantum numbers arise naturally from the mathematics used to describe the possible states of an electron in an atom. The four
quantum numbers, the principal quantum number (n), the angular momentum quantum number (E), the magnetic quantum
number (me), and the spin quantum number (m3) have strict rules which govern the possible values.
Identify all allowable combinations of quantum numbers for an electron.
3, e = 1, me = 1, m,
n =
п %3D 5, € 3D 5, тe
0, ms
2
n = 2, € = 1, me
0, ms
-1
5, e = 2, me =
2, ms
n =
n =
-1, mẹ =
1,
ms
n = 4, € = 0, mẹ = 1, ms
1/2
1/2
1/2
3,
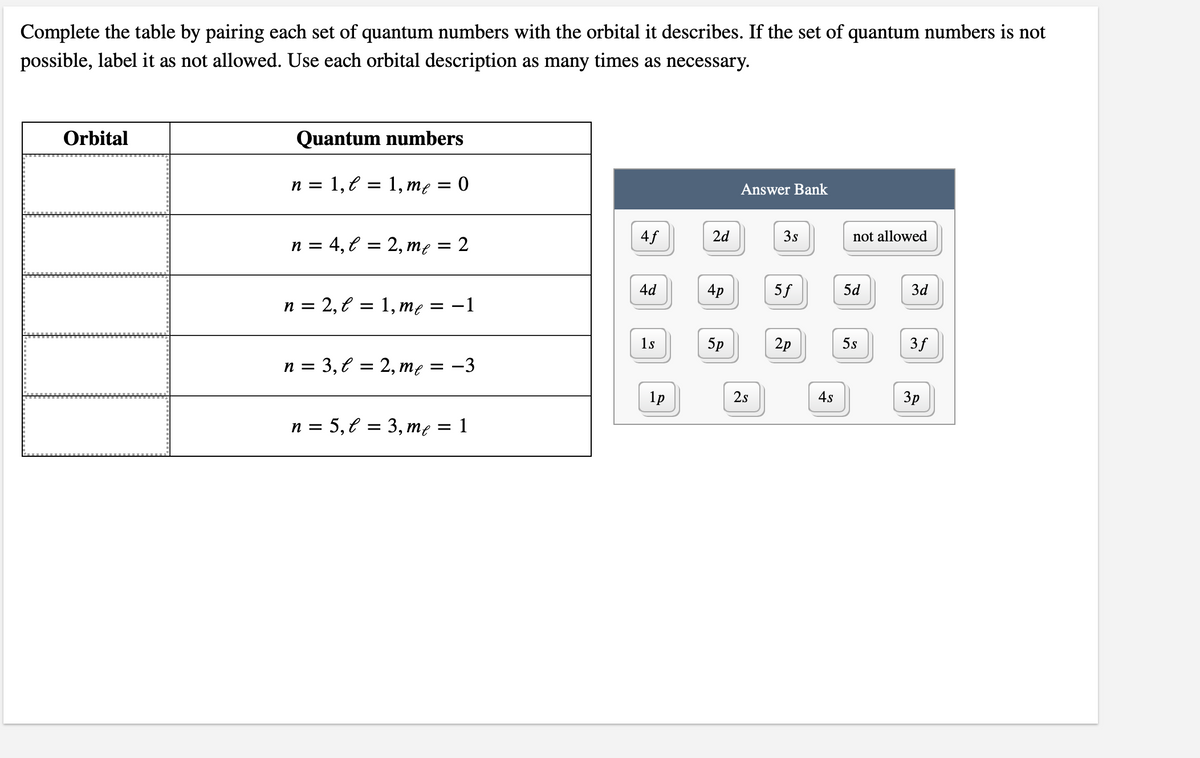
Transcribed Image Text:Complete the table by pairing each set of quantum numbers with the orbital it describes. If the set of quantum numbers is not
possible, label it as not allowed. Use each orbital description as many times as necessary.
Orbital
Quantum numbers
1, € =
3D 1, те 3D 0
n =
Answer Bank
n = 4, € = 2, me
4f
2d
3s
not allowed
4d
4p
5f
5d
3d
n = 2, € = 1, me
= -1
1s
5p
2p
5s
3f
n = 3,€ = 2, mę = -3
1p
2s
4s
3p
n = 5,€ = 3, me
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning