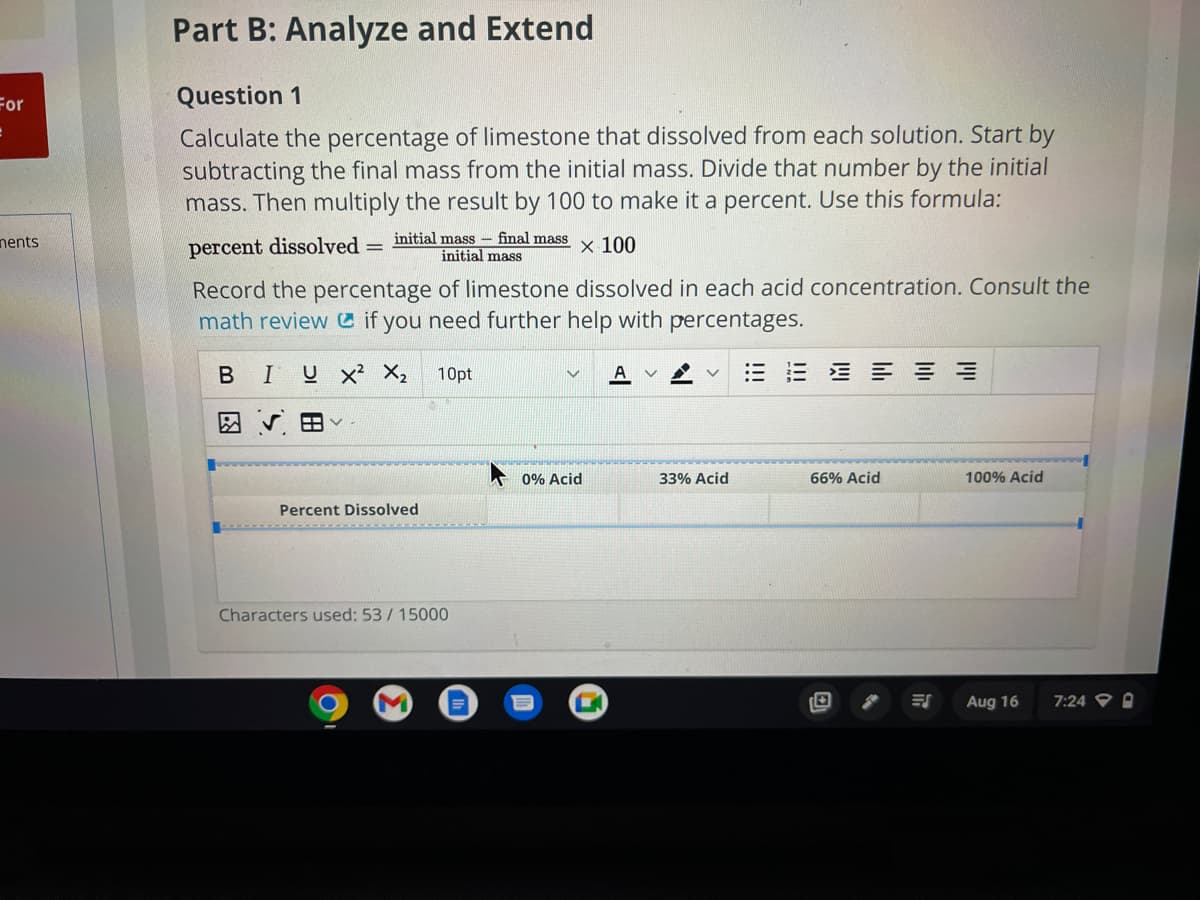
Question 1 Calculate the percentage of limestone that dissolved from each solution. Start by subtracting the final mass from the initial mass. Divide that number by the initial mass. Then multiply the result by 100 to make it a percent. Use this formula: percent dissolved initial mass - final mass x 100 initial mass Record the percentage of limestone dissolved in each acid concentration. Consult the math review if you need further help with percentages. B I UX² X₂ 10pt HV Percent Dissolved V A 0% Acid 33% Acid 三三三三 66% Acid 100% Acid
Question 1 Calculate the percentage of limestone that dissolved from each solution. Start by subtracting the final mass from the initial mass. Divide that number by the initial mass. Then multiply the result by 100 to make it a percent. Use this formula: percent dissolved initial mass - final mass x 100 initial mass Record the percentage of limestone dissolved in each acid concentration. Consult the math review if you need further help with percentages. B I UX² X₂ 10pt HV Percent Dissolved V A 0% Acid 33% Acid 三三三三 66% Acid 100% Acid
Applications and Investigations in Earth Science (9th Edition)
9th Edition
ISBN:9780134746241
Author:Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Chapter1: The Study Of Minerals
Section: Chapter Questions
Problem 1LR
Related questions
Question
Graph the percentages you obtained in question 1. Use the circle tool to place your points.
Transcribed Image Text:For
e
ments
Part B: Analyze and Extend
Question 1
Calculate the percentage of limestone that dissolved from each solution. Start by
subtracting the final mass from the initial mass. Divide that number by the initial
mass. Then multiply the result by 100 to make it a percent. Use this formula:
percent dissolved:
Record the percentage of limestone dissolved in each acid concentration. Consult the
math review if you need further help with percentages.
B I U X² X₂ 10pt
-
initial mass- final mass
initial mass
Percent Dissolved
Characters used: 53/ 15000
M
x 100
0% Acid
O
A
33% Acid
66% Acid
ES
3
100% Acid
Aug 16
7:24
Transcribed Image Text:Fill:
Percent Dissolved
Question 3
Line:
6%
5%
4%
3%
2%
1%
0%
Width: 2 pt
Percent Dissolved vs. Acid Concentration
0%
33%
Acid Concentration
66%
100%
DCE
=S
Aug 16
7:26
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Applications and Investigations in Earth Science …
Earth Science
ISBN:
9780134746241
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Exercises for Weather & Climate (9th Edition)
Earth Science
ISBN:
9780134041360
Author:
Greg Carbone
Publisher:
PEARSON

Environmental Science
Earth Science
ISBN:
9781260153125
Author:
William P Cunningham Prof., Mary Ann Cunningham Professor
Publisher:
McGraw-Hill Education

Applications and Investigations in Earth Science …
Earth Science
ISBN:
9780134746241
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Exercises for Weather & Climate (9th Edition)
Earth Science
ISBN:
9780134041360
Author:
Greg Carbone
Publisher:
PEARSON

Environmental Science
Earth Science
ISBN:
9781260153125
Author:
William P Cunningham Prof., Mary Ann Cunningham Professor
Publisher:
McGraw-Hill Education

Earth Science (15th Edition)
Earth Science
ISBN:
9780134543536
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Environmental Science (MindTap Course List)
Earth Science
ISBN:
9781337569613
Author:
G. Tyler Miller, Scott Spoolman
Publisher:
Cengage Learning

Physical Geology
Earth Science
ISBN:
9781259916823
Author:
Plummer, Charles C., CARLSON, Diane H., Hammersley, Lisa
Publisher:
Mcgraw-hill Education,