QUESTION 10 A four-cylinder, 3.6-liter, four-stroke cycles Sl engine operates at 2700 RPM with a volumetric efficiency of 87 %. The fuel used is methyl alcohol. The chemical reaction of methyl alcohol in a Stoichiometric condition is: CH3OH + 1.5(02 + 3.76N2) - CO2 + 2H20 + 1.5(3.76N2) The chemical reaction of methyl alcohol in an equivalence ratio of o = 1.23 is: CH3OH + m(02 + 3.76N2) → NCO2 + pCO + 2H20 + m(3.76N2) During combustion, all hydrogen is converted to water, and all carbon is converted to CO2 and CO. The density of standard air is 2.25 kg/m3, The atomic masses are as follows: He1, C-12, 0-16, and N=14, Calculate the following: m is n is p is Mole fraction of CO in the exhaust is The mass flow rate of air into the engine is gram/s.
QUESTION 10 A four-cylinder, 3.6-liter, four-stroke cycles Sl engine operates at 2700 RPM with a volumetric efficiency of 87 %. The fuel used is methyl alcohol. The chemical reaction of methyl alcohol in a Stoichiometric condition is: CH3OH + 1.5(02 + 3.76N2) - CO2 + 2H20 + 1.5(3.76N2) The chemical reaction of methyl alcohol in an equivalence ratio of o = 1.23 is: CH3OH + m(02 + 3.76N2) → NCO2 + pCO + 2H20 + m(3.76N2) During combustion, all hydrogen is converted to water, and all carbon is converted to CO2 and CO. The density of standard air is 2.25 kg/m3, The atomic masses are as follows: He1, C-12, 0-16, and N=14, Calculate the following: m is n is p is Mole fraction of CO in the exhaust is The mass flow rate of air into the engine is gram/s.
Automotive Technology: A Systems Approach (MindTap Course List)
6th Edition
ISBN:9781133612315
Author:Jack Erjavec, Rob Thompson
Publisher:Jack Erjavec, Rob Thompson
Chapter9: Automotive Engine Designs And Diagnosis
Section: Chapter Questions
Problem 15RQ: Which of the following is an expression of how much of the heat formed during the combustion process...
Related questions
Question
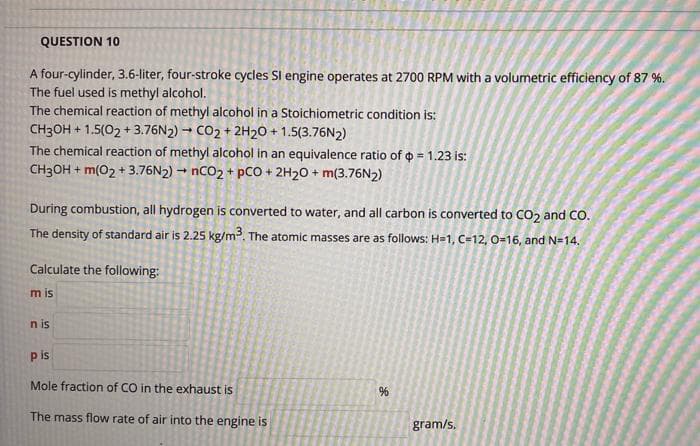
Transcribed Image Text:QUESTION 10
A four-cylinder, 3.6-liter, four-stroke cycles Sl engine operates at 2700 RPM with a volumetric efficiency of 87 %.
The fuel used is methyl alcohol.
The chemical reaction of methyl alcohol in a Stoichiometric condition is:
CH3OH + 1.5(02 + 3.76N2) - CO2 + 2H20 + 1.5(3.76N2)
The chemical reaction of methyl alcohol in an equivalence ratio of o = 1.23 is:
CH3OH + m(02 + 3.76N2) → NCO2 + pCO + 2H20 + m(3.76N2)
During combustion, all hydrogen is converted to water, and all carbon is converted to CO2 and CO.
The density of standard air is 2.25 kg/m. The atomic masses are as follows: H=1, C=12, 0-16, and N=14.
Calculate the following:
m is
n is
p is
Mole fraction of CO in the exhaust is
The mass flow rate of air into the engine is
gram/s.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Automotive Technology: A Systems Approach (MindTa…
Mechanical Engineering
ISBN:
9781133612315
Author:
Jack Erjavec, Rob Thompson
Publisher:
Cengage Learning

Automotive Technology: A Systems Approach (MindTa…
Mechanical Engineering
ISBN:
9781133612315
Author:
Jack Erjavec, Rob Thompson
Publisher:
Cengage Learning