Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 62QAP: Mustard gas, used in chemical warfare in World War I, has been found to be an effective agent in the...
Related questions
Question
100%
Question 13
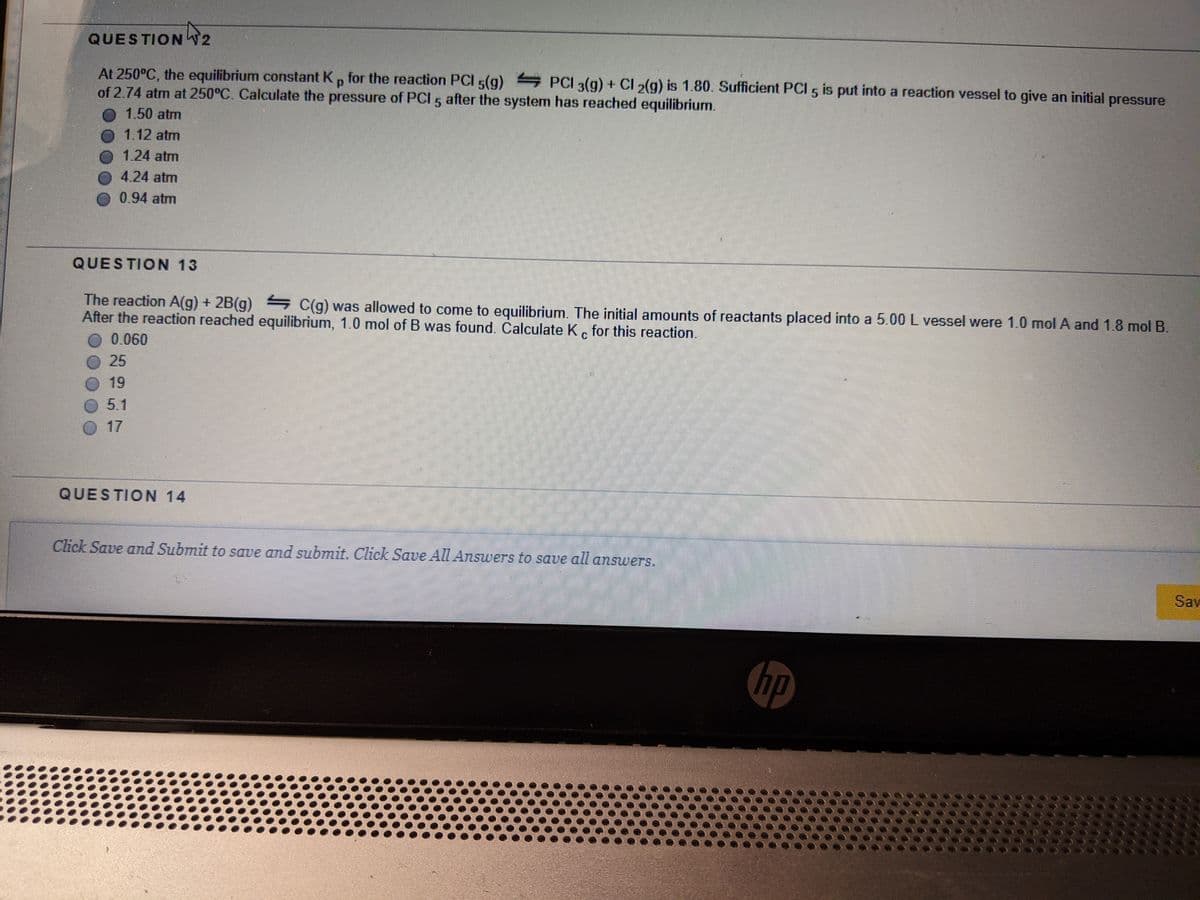
Transcribed Image Text:QUESTION T2
At 250°C, the equilibrium constant K, for the reaction PCI 5(g) PCI 3(g) + CI 2(g) is 1.80. Sufficient PCI 5 is put into a reaction vessel to give an initial pressure
of 2.74 atm at 250°C. Calculate the pressure of PCI 5 after the system has reached equilibrium.
1.50 atm
1.12 atm
O 1.24 atm
4.24 atm
0.94 atm
QUESTION 13
The reaction A(g) + 2B(g) C(g) was allowed to come to equilibrium. The initial amounts of reactants placed into a 5.00 L vessel were 1.0 mol A and 1.8 mol B.
After the reaction reached equilibrium, 1.0 mol of B was found. Calculate K. for this reaction.
0.060
25
19
5.1
17
QUESTION 14
Click Save and Submit to save and submit. Click SaUe All Answers to save all answers,
Sav
hp
Expert Solution
Step 1
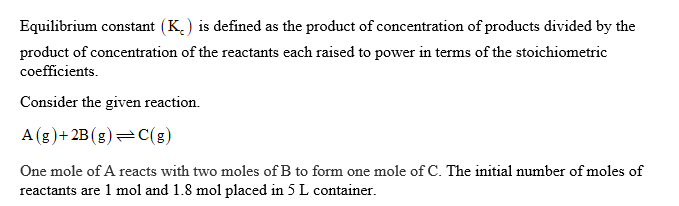
Step 2

Step 3
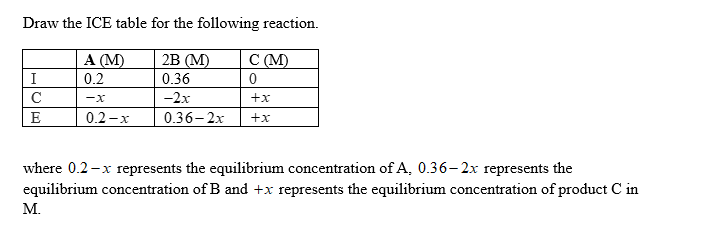
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax